No products in the cart.
Ibuprofen, gel 5% 50 g
€4.60 €3.83
Description
Pharmacotherapeutic group
Non-steroidal anti-inflammatory drug (NSAID).
ATC code
M02AA13
Pharmacological properties
The drug in the dosage form gel for external use is easy to apply and quickly absorbed into the skin.
Pharmacodynamics
Ibuprofen belongs to the group of non-steroidal anti-inflammatory drugs. It has a dual action: analgesic and anti-inflammatory. Ibuprofen, as a derivative of propionic acid, non-selectively blocks cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2), and as a result inhibits the synthesis of prostaglandins – mediators of pain, inflammation and hyperthermia.
In application to the skin the gel has an additional cooling effect due to rapid evaporation of the ethyl alcohol contained in its composition.
Pharmacokinetics
After application to the skin, ibuprofen is detectable in the epidermis and dermis after 24 hours. Maximum concentration in blood plasma of ibuprofen when administered topically is 5% of the maximum concentration level when using oral forms of ibuprofen. There is practically no clinically significant systemic absorption.
It is metabolized in the liver. It is excreted by the kidneys (not more than 1% unchanged) and, to a lesser extent, in the bile.
Indications
Indications
As a local analgesic and anti-inflammatory agent for conditions such as muscle pain, back pain, arthritis, pain from ligament injuries and sprains, sports injuries and neuralgia.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group
Non-steroidal anti-inflammatory drug (NSAID).
ATX code
M02AA13
Pharmacological properties
The drug in gel dosage form for external use is easily applied and quickly absorbed into the skin.
Pharmacodynamics
Ibuprofen belongs to the group of non-steroidal anti-inflammatory drugs. Has a double effect: analgesic and anti-inflammatory. Ibuprofen, being a derivative of propionic acid, indiscriminately blocks cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2), as a result of which it inhibits the synthesis of prostaglandins – mediators of pain, inflammation and hyperthermic reaction.
When applied to the skin, the gel has an additional cooling effect due to the rapid evaporation of the ethyl alcohol contained in the composition.
Pharmacokinetics
After application to the skin, ibuprofen is detected in the epidermis and dermis after 24 hours. The maximum plasma concentration of ibuprofen when applied topically is 5% of the maximum concentration level when using oral forms of ibuprofen. There is practically no clinically significant systemic absorption.
Metabolized in the liver. It is excreted by the kidneys (no more than 1% unchanged) and, to a lesser extent, with bile.
Special instructions
Special instructions
Avoid contact with eyes, lips and other mucous membranes, inflamed or damaged areas of skin.
Do not use in combination with an occlusive (sealed) medical dressing.
Avoid excessive exposure to sunlight on the area of application of the drug.
Impact on the ability to drive vehicles and machinery
Does not affect activities that require increased concentration and speed of psychomotor reactions (driving vehicles or operating machinery).
Active ingredient
Active ingredient
Ibuprofen
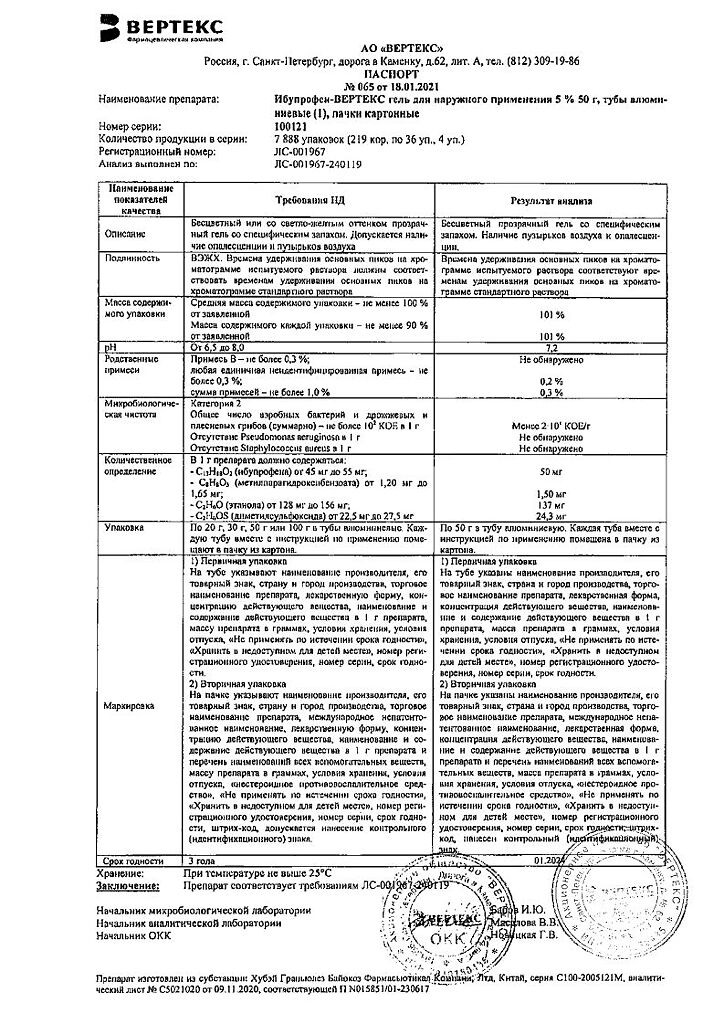
Composition
Composition
1 g of gel contains:
active ingredient: ibuprofen – 50.0 mg;
excipients: ethyl alcohol 95% (ethanol 95%) – 150.0 mg; propylene glycol – 100.0 mg; trolamine (triethanolamine) – 60.0 mg; dimethyl sulfoxide – 25.0 mg; carbomer – 15.0 mg; methyl parahydroxybenzoate (methylparaben) – 1.5 mg; lavender oil – 1.0 mg; orange flower oil (neroli oil) – 0.5 mg; purified water – up to 1.0 g.
Pregnancy
Pregnancy
The use of the drug is contraindicated in the third trimester of pregnancy.
You should avoid using the drug in the first and second trimesters of pregnancy; if you need to use the drug, you should consult your doctor.
There is evidence that ibuprofen can pass into breast milk in small quantities without any adverse effects on the health of the nursing infant, so there is usually no need to stop breastfeeding when used for short periods of time.
If long-term use of the drug is necessary, you should consult a doctor to decide whether to stop breastfeeding for the period of use of the drug.
Contraindications
Contraindications
hypersensitivity to ibuprofen or other components included in the drug;
a history of hypersensitivity reactions (bronchial asthma, rhinitis, Quincke’s edema, urticaria) in response to the use of acetylsalicylic acid or other NSAIDs;
violations of the integrity of the skin at the site of application of the drug (including infected wounds and abrasions, weeping dermatitis, eczema);
children under 14 years of age;
pregnancy III trimester.
With caution
If you have the conditions listed in this section, you should consult a doctor before using the drug.
Bronchial asthma or allergic diseases in the acute stage or in history – possible development of bronchospasm, renal failure, liver failure, gastric ulcer, including a history of it, enteritis, colitis, hemorrhagic diathesis, pregnancy I-II trimester, breastfeeding period.
Side Effects
Side Effects
The incidence of adverse reactions was assessed based on the following criteria:
very often ≥ 1/10;
often from ≥ 1/100 to < 1/10;
infrequently from ≥ 1/1000 to < 1/100;
rarely from ≥ 1/10000 to < 1/1000;
very rarely >1/10000;
frequency unknown (frequency cannot be determined from available data). The following adverse reactions were observed with short-term use of ibuprofen at a dose not exceeding 500 mg/day. When treating chronic conditions and with long-term use, other adverse reactions may occur.
Immune system disorders:
frequency unknown – hypersensitivity reactions: nonspecific allergic reactions and anaphylactic reactions, reactions from the respiratory tract (bronchial asthma, including its exacerbation, bronchospasm, shortness of breath, dyspnea), skin reactions (itching, urticaria, purpura, Quincke’s edema, exfoliative and bullous dermatoses, including toxic epidermal necrolysis syndrome Lyell), Stevens-Johnson syndrome (erythema multiforme).
Gastrointestinal disorders:
frequency unknown – abdominal pain, dyspepsia.
Renal and urinary tract disorders:
frequency unknown – renal dysfunction.
If side effects occur, you should immediately stop using the drug and consult a doctor.
Interaction
Interaction
Drug interactions between ibuprofen in the form of a gel for external use and other drugs have not been described.
Use with caution simultaneously with the following drugs: anticoagulants and thrombolytic drugs, antihypertensive drugs, acetylsalicylic acid, other NSAIDs.
It should be borne in mind that even with topical use of ibuprofen, its systemic effect cannot be completely excluded and, theoretically, side effects may increase when the drug is used simultaneously with other NSAIDs.
Overdose
Overdose
The likelihood of an accidental overdose of the drug in gel form is minimal.
In children, overdose symptoms may occur after ingestion of a dose exceeding 400 mg/kg body weight. In adults, the dose-dependent effect of overdose is less pronounced. The half-life of the drug in case of overdose is 1.5-3 hours.
If the drug is accidentally taken orally, the following symptoms may occur: headache, vomiting, decreased blood pressure.
Treatment
Gastric lavage (only within an hour after administration), taking activated charcoal, alkaline drinking, forced diuresis, symptomatic therapy.
Storage conditions
Storage conditions
Store at a temperature not exceeding 25 °C. Keep out of the reach of children.
Shelf life
Shelf life
3 years.
Do not use after expiration date.
Manufacturer
Manufacturer
Vertex, Russia
Additional information
| Shelf life | 3 years. Do not use after the expiration date. |
|---|---|
| Conditions of storage | Store at the temperature not more than 25 °С. Keep out of reach of children. |
| Manufacturer | Vertex, Russia |
| Medication form | gel for external use |
| Brand | Vertex |
Other forms…
Related products
Buy Ibuprofen, gel 5% 50 g with delivery to USA, UK, Europe and over 120 other countries.