Subtotal: €202.54
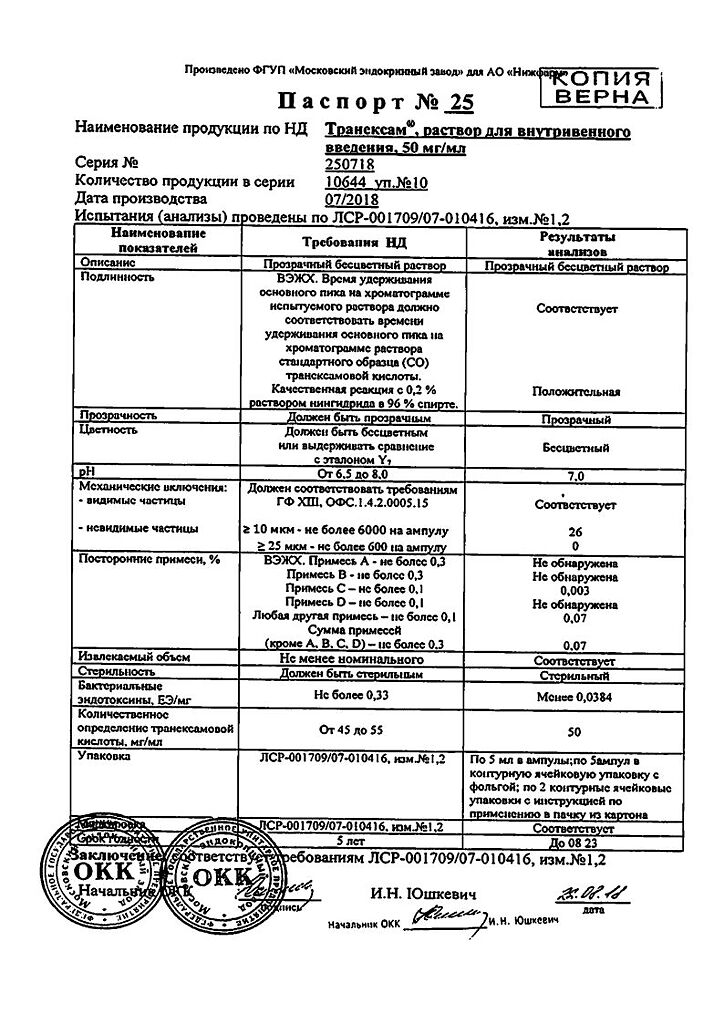
Tranexam, 50 mg/ml 5 ml 10 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Tranexamic has a hemostatic, anti-inflammatory, anti-allergic effect.
Tranexamic acid is an antifibrinolytic agent specifically inhibiting the activation of profibrinolysin (plasminogen) and its conversion into fibrinolysin (plasmin).
It has local and systemic hemostatic action in bleeding associated with increased fibrinolysis as well as anti-inflammatory, anti-allergic, anti-infective and
anti-tumor action due to suppression of formation of kinins and other active peptides involved in allergic and inflammatory reactions.
The intrinsic analgesic activity of tranexamic acid was confirmed in the experiment, as well as the supersumptive andotensive effect with respect to the analgesic activity of opiates.
Pharmacokinetics
The distribution in tissues is relatively uniform (exception is cerebrospinal fluid, where the concentration is 1/10 of the plasma concentration); penetrates through the placental and blood-brain barrier, into breast milk (about 1% of the concentration in maternal plasma).
Detected in seminal fluid, where it reduces fibrinolytic activity, but does not affect sperm migration. The initial volume of distribution is 9-12 liters.
The binding to plasma proteins (profibrinolysin) is less than 3%. In the blood about 3% is bound to protein (plasminogen).
The concentration in cerebrospinal fluid is 1/10 of plasma. Total renal clearance is equal to plasma clearance. Antifibrinolytic concentration in various tissues persists for 17 hours, in plasma – up to 7-8 hours.
A small part is metabolized. The concentration-time curve has a three-phase shape with a half-life of -2 hours in the terminal phase. Total renal clearance is equal to plasma clearance (7 l/h).
Extracted by the kidneys (main route – glomerular filtration) – more than 95% unchanged in the first 12 hours.
Two metabolites of tranexamic acid have been identified: N-acetylated and deaminated derivatives. There is a risk of cumulation of tranexamic acid in impaired renal function.
Indications
Prevention and treatment of bleeding caused by generalized or local fibrinolysis in adults and children aged 1 year and older, including: menorrhagia and metrorrhagia; gastrointestinal bleeding; bleeding after surgical interventions on the prostate gland and urinary tract; bleeding during surgical interventions in the nasal cavity, mouth and pharynx (adenoidectomy, tonsillectomy, tooth extraction); bleeding during thoracic, abdominal and other extensive surgical interventions (including cardiac surgery); obstetric and gynecological bleeding (including bleeding during gynecological surgical interventions); bleeding caused by the use of fibrinolytic drugs.
Pharmacological effect
Tranexam has a hemostatic, anti-inflammatory, antiallergic effect.
Tranexamic acid is an antifibrinolytic agent that specifically inhibits the activation of profibrinolysin (plasminogen) and its conversion to fibrinolysin (plasmin).
It has a local and systemic hemostatic effect in case of bleeding associated with increased fibrinolysis, as well as anti-inflammatory, anti-allergic, anti-infective and
antitumor effects due to suppression of the formation of kinins and other active peptides involved in allergic and inflammatory reactions.
The experiment confirmed the intrinsic analgesic activity of tranexamic acid, as well as the super-total ioteniating effect on the analgesic activity of opiates.
Pharmacokinetics
Distributed relatively evenly in tissues (with the exception of cerebrospinal fluid, where the concentration is 1/10 of the plasma concentration); penetrates the placental and blood-brain barrier into breast milk (about 1% of the concentration in maternal plasma).
It is found in seminal fluid, where it reduces fibrinolytic activity, but does not affect sperm migration. The initial volume of distribution is 9-12 l.
Communication with plasma proteins (profibrinolysin) – less than 3%. In the blood, about 3% is associated with protein (plasminogen).
The concentration in the cerebrospinal fluid is 1/10 of the plasma concentration. Total renal clearance is equal to plasma clearance. Antifibrinolytic concentration in various tissues lasts 17 hours, in plasma – up to 7-8 hours.
A small part is metabolized. The concentration-time curve has a triphasic shape with a half-life in the terminal phase of -2 hours. Total renal clearance is equal to plasma (7 l/h).
Excreted by the kidneys (the main route is glomerular filtration) – more than 95% unchanged during the first 12 hours.
Two metabolites of tranexamic acid have been identified: N-acetylated and deaminated derivatives. In case of impaired renal function, there is a risk of accumulation of tranexamic acid.
Special instructions
Before starting and during treatment with tranexamic acid, it is necessary to consult an ophthalmologist (determining visual acuity, color vision, condition of the fundus). If visual impairment occurs during treatment with tranexamic acid, the drug must be discontinued. Tranexamic acid preparations should be used with caution in hematuria caused by diseases of the renal parenchyma, since intravascular fibrin deposition is often observed in these conditions, which can aggravate renal damage. In addition, in cases of massive bleeding of any etiology from the upper urinary tract, antifibrinolytic therapy increases the risk of blood clots in the renal pelvis and/or ureter and, accordingly, secondary mechanical obstruction of the urinary tract and the development of anuria. Although clinical studies have not revealed a significant increase in the incidence of thrombosis, the risk of thrombotic complications cannot be completely excluded. Cases of the development of venous and arterial thrombosis and thromboembolism in patients receiving tranexamic acid have been described. In addition, cases of occlusion of the central retinal artery and central retinal vein have been reported. Several patients developed intracranial thrombosis during treatment with tranexamic acid. Accordingly, in patients with a high risk of developing thrombosis (history of thromboembolic complications, cases of thromboembolism in relatives, verified diagnosis of thrombophilia), tranexamic acid should be used only if absolutely necessary and under strict medical supervision. Before using tranexamic acid, an examination should be performed to identify risk factors for thromboembolic complications. The presence of blood in cavities, such as the pleural cavity, joint cavities and urinary tract (including the renal pelvis and bladder) can lead to the formation of an “insoluble clot” due to extravascular coagulation, which may be resistant to physiological fibrinolysis. Patients with irregular menstrual bleeding should not be prescribed tranexamic acid until the cause of dysmenorrhea is determined. If the amount of menstrual bleeding is inadequately reduced during treatment with tranexamic acid, alternative treatment should be considered. The effectiveness and safety of tranexamic acid preparations in the treatment of menorrhagia in patients under 16 years of age have not been established. Tranexamic acid should be used with caution in women concomitantly taking combined oral contraceptives due to the increased risk of thrombosis (See section “Interaction with other drugs”). In patients with DIC who require treatment with tranexamic acid, therapy should be carried out under the close supervision of a physician experienced in treating this disease. Due to the lack of adequate clinical studies, the simultaneous use of tranexamic acid with anticoagulants should be under the close supervision of a specialist experienced in the treatment of bleeding disorders. If vision impairment occurs while taking tranexamic acid, you should stop taking the drug and consult a doctor.
Active ingredient
Tranexamic acid
Active components
Tranexamic acid
Composition
1 liter of solution for intravenous administration contains
Active ingredient:
Tranexamic acid 50 g;
Excipients:
Water for injections – up to 1 l
Pregnancy
In preclinical studies, tranexamic acid did not have a teratogenic effect. Adequate and strictly controlled studies of the effectiveness and safety of tranexamic acid in pregnant women have not been conducted. Tranexamic acid crosses the placenta and may be present in cord blood in concentrations close to maternal levels. Because animal reproduction studies do not always predict reactions in humans, tranexamic acid should be used during pregnancy only when absolutely necessary. Tranexamic acid passes into breast milk (the concentration of the drug in milk is about 1% of the concentration in the mother’s blood plasma). The development of an antifibrinolytic effect in an infant is unlikely. However, caution should be exercised when using tranexamic acid in nursing mothers.
Contraindications
Hypersensitivity to tranexamic acid or other components of the drug; Severe chronic renal failure (glomerular filtration rate [GFR] less than 30 mg/ml/1.73 m2) due to the risk of accumulation; Venous or arterial thrombosis currently or in history (deep vein thrombosis of the legs, pulmonary embolism, thrombosis of intracranial vessels, etc.) if simultaneous anticoagulant therapy is impossible; Fibrinolysis due to consumption coagulopathy (hypocoagulable stage of disseminated intravascular coagulation syndrome [DIC]); history of seizures; Acquired color vision impairment; Subarachnoid hemorrhage (due to the risk of developing cerebral edema, ischemia and cerebral infarction); Treatment of menorrhagia in patients under the age of 16 years (no experience of use); Children under 1 year of age (no experience of use).
Side Effects
The incidence of adverse drug reactions is determined in accordance with the WHO classification: very often (≥1/10), often (≥1/100, 1/10), infrequently (≥1/1000, 1/100), rarely (≥1/10000, 1/1000), very rarely (1/10000), frequency unknown (cannot be determined from the available data). Gastrointestinal disorders: often – nausea, vomiting, diarrhea (symptoms disappear when the dose is reduced). Disorders of the skin and subcutaneous tissues: rarely – allergic skin reactions, including allergic dermatitis. Disorders of the organ of vision: rarely – visual disturbances, including impaired color perception, thrombosis of retinal vessels. Vascular disorders: rarely – thromboembolic complications, marked decrease in blood pressure (usually due to excessively rapid intravenous administration); very rarely – arterial and venous thrombosis of various locations; frequency unknown – acute myocardial infarction, cerebral artery thrombosis, carotid artery thrombosis, stroke, deep vein thrombosis of the legs, pulmonary embolism, renal artery thrombosis with the development of cortical necrosis and acute renal failure, occlusion of coronary artery bypass graft, thrombosis of the central artery and retinal vein. Immune system disorders: very rarely – hypersensitivity reactions, including anaphylactic shock. Nervous system disorders: rarely – dizziness, convulsions.
Interaction
Pharmaceutically incompatible with blood products, solutions containing penicillin, urokinase, hypertensive drugs (norepinephrine deoxyepinephrine hydrochloride, metharmine bitartrate), tetracyclines, dipyridamole, diazepam.
Hemostatic drugs and hemocoagulase potentiate the activation of thrombus formation.
Complete set of goods
Solution for intravenous administration 50 mg/ml. 5 ml in neutral glass ampoules. 5 ampoules each in a blister pack made of polyvinyl chloride film or in a blister pack made of polyvinyl chloride film and printed varnished aluminum foil. 1 or 2 blister packs along with instructions for use in a cardboard pack. Scarifiers or ampoule knives are placed in the pack. When packaging ampoules with notches, rings and break points, scarifiers or ampoule knives are not used. Packaging “for hospitals” 20, 50 or 100 foil-coated blister packs with 20, 50 and 100 instructions for use, scarifiers or ampoule knives in cardboard boxes or corrugated cardboard boxes. When packaging ampoules with notches, rings and break points, scarifiers or ampoule knives are not used.
Storage conditions
At a temperature not exceeding 25° C. Keep out of the reach of children.
Shelf life
5 years. Do not use after the expiration date stated on the package.
Manufacturer
Moscow Endocrine Plant, Russia
| Shelf life | 5 years. Do not use after the expiration date printed on the package. |
|---|---|
| Conditions of storage | At a temperature not exceeding 25 ° C. Keep out of reach of children. |
| Manufacturer | Moscow Endocrine Plant, Russia |
| Medication form | solution |
| Brand | Moscow Endocrine Plant |
Other forms…
Related products
Buy Tranexam, 50 mg/ml 5 ml 10 pcs with delivery to USA, UK, Europe and over 120 other countries.