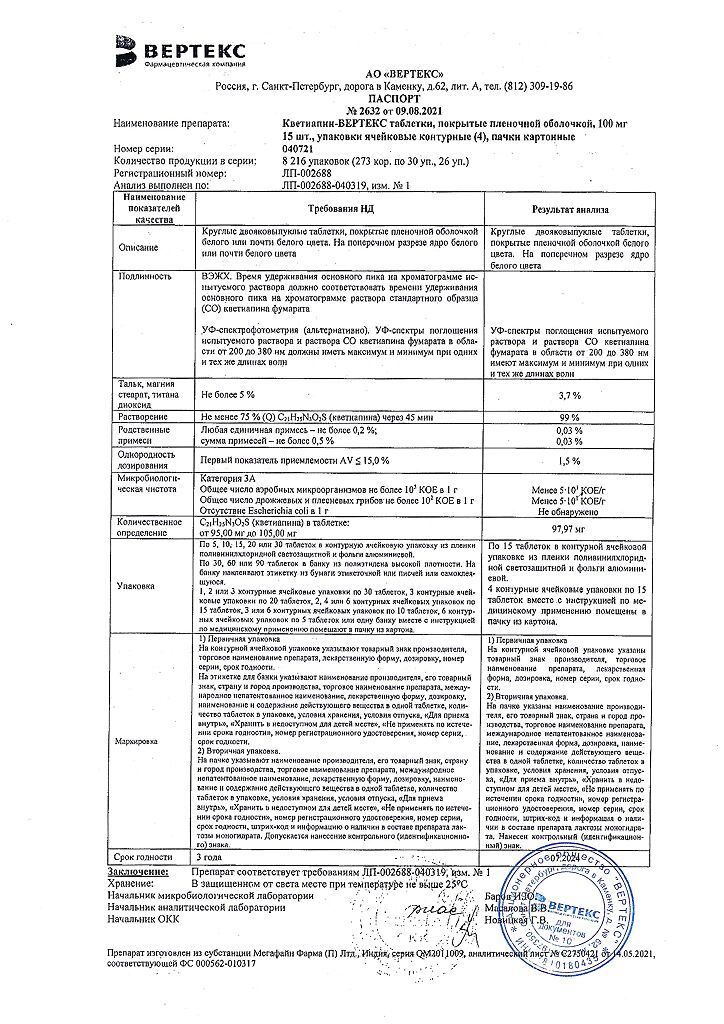
No products in the cart.
Quetiapine, 100 mg 60 pcs
€30.52 €26.45
Out of stock
(E-mail when Stock is available)
Description
For the treatment of:
- schizophrenia;
- manic episodes in the structure of bipolar disorder;
- depressive episodes of moderate to severe severity in the structure of bipolar disorder.
The drug is not indicated for prevention of manic and depressive episodes.
To treat:
- schizophrenia;
- manic episodes in the structure of bipolar disorder;
- depressive episodes of moderate to severe severity in the structure of bipolar disorder.
The drug is not indicated for prevention of manic and depressive episodes.
To treat:
- schizophrenia;
- manic episodes in the structure of bipolar disorder;
- depressive episodes of moderate to severe severity in the structure of bipolar disorder.
The drug is not indicated for prevention of manic and depressive episodes.
To treat:
- schizophrenia;
- manic episodes in the structure of bipolar disorder;
- depressive episodes of moderate to severe severity in the structure of bipolar disorder.
The drug is not indicated for prevention of manic and depressive episodes.
.
.
Indications
Indications
For treatment:
schizophrenia;
manic episodes in the structure of bipolar disorder;
depressive episodes of moderate to severe severity in the structure of bipolar disorder.
The drug is not indicated for the prevention of manic and depressive episodes.
For treatment:
schizophrenia;
manic episodes in the structure of bipolar disorder;
depressive episodes of moderate to severe severity in the structure of bipolar disorder.
The drug is not indicated for the prevention of manic and depressive episodes.
For treatment:
schizophrenia;
manic episodes in the structure of bipolar disorder;
depressive episodes of moderate to severe severity in the structure of bipolar disorder.
The drug is not indicated for the prevention of manic and depressive episodes.
For treatment:
schizophrenia;
manic episodes in the structure of bipolar disorder;
depressive episodes of moderate to severe severity in the structure of bipolar disorder.
The drug is not indicated for the prevention of manic and depressive episodes.
.
Special instructions
Special instructions
There was no relationship between taking quetiapine and an increase in the QTc interval. However, when using quetiapine simultaneously with drugs that prolong the QTc interval, caution must be exercised.
In children, adolescents and young people (under 24 years of age) with depression and other mental disorders, antidepressants, compared with placebo, increase the risk of suicidal thoughts and suicidal behavior. Therefore, when prescribing antidepressants in children, adolescents and young adults (under 24 years of age), the risk of suicide should be weighed against the benefits of their use.
In short-term studies, the risk of suicide did not increase in people over 24 years of age, but it decreased slightly in people over 65 years of age. Any depressive disorder itself increases the risk of suicide. Therefore, during treatment with antidepressants, all patients should be monitored for early detection of disturbances or changes in behavior, as well as suicidality.
During the treatment period, care must be taken when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Quetiapine
Composition
Composition
active substance:
quetiapine fumarate (quetiapine hemifumarate) in terms of quetiapine – 100,000 mg;
excipients:
microcrystalline cellulose – 34.870 mg,
lactose monohydrate – 18,000 mg,
sodium carboxymethyl starch (sodium starch glycolate, type A) – 14,000 mg,
povidone K-30 – 8,000 mg,
talc – 5,000 mg,
colloidal silicon dioxide – 3,000 mg,
magnesium stearate – 2,000 mg;
film cover:
[hypromellose – 3.600 mg, talc – 1.200 mg, titanium dioxide – 0.660 mg, macrogol 4000 (polyethylene glycol 4000) – 0.540 mg] or [dry film coating mixture containing hypromellose (60%), talc (20%), titanium dioxide (11%), macrogol 4000 (polyethylene glycol 4000) (9%)] – 6,000 mg.
Contraindications
Contraindications
hypersensitivity to any of the components of the drug, including lactase deficiency, glucose-galactose malabsorption and galactose intolerance;
combined use with cytochrome P450 inhibitors, such as azole antifungals, erythromycin, clarithromycin and nefazodone, as well as HIV protease inhibitors (see section “Interaction with other drugs”);
age up to 18 years.
With caution
in patients with cardiovascular and cerebrovascular diseases or other conditions predisposing to arterial hypotension;
old age;
liver failure;
history of seizures;
risk of stroke;
risk of developing aspiration pneumonia.
Side Effects
Side Effects
From the nervous system: drowsiness, dizziness, headache, anxiety, asthenia, hostility, agitation, insomnia, akathisia, tremor, convulsions, depression, paresthesia, neuroleptic malignant syndrome (hyperthermia, muscle rigidity, altered mental status, lability of the autonomic nervous system, increased creatine phosphokinase activity).
From the cardiovascular system: orthostatic hypotension (accompanied by dizziness), tachycardia, fainting, prolongation of the QT interval (no relationship between the use of quetiapine and a constant increase in QTc has been identified).
From the digestive system: dryness of the oral mucosa, nausea, vomiting, abdominal pain, diarrhea or constipation, increased activity of liver transaminases.
From the respiratory system: pharyngitis, rhinitis.
Allergic reactions: skin rash, eosinophilia.
Laboratory indicators: leukopenia, hypercholesterolemia, hypertriglyceridemia, decreased thyroxine concentration (first 4 weeks).
Other: lower back pain, chest pain, low-grade fever, weight gain (mainly in the first weeks of treatment), myalgia, dry skin, blurred vision.
Interaction
Interaction
With simultaneous administration of drugs that have a strong inhibitory effect on CYP3A4 (such as azole antifungals and macrolide antibiotics), the plasma concentration of quetiapine may increase.
In such cases, lower doses of quetiapine should be used. Particular attention should be paid to elderly and debilitated patients. It is necessary to assess the risk-benefit ratio individually for each patient.
Inducers of liver microsomal systems (phenytoin, etc.), thioridazine increase the clearance of quetiapine, inhibitors of liver microsomal systems reduce it; at the same time, the simultaneous administration of quetiapine and antidepressants – imipramine (inhibitor of CYP2D6) or fluoxetine (inhibitor of CYP3A4 and CYP2D6) does not have a significant effect on its pharmacokinetics.
The pharmacokinetics of quetiapine does not change significantly when administered concomitantly with the antipsychotic drugs risperidone or haloperidol.
Does not cause induction of liver enzyme systems involved in the metabolism of antipyrine.
The pharmacokinetics of lithium preparations does not change with simultaneous administration of quetiapine.
There were no clinically significant changes in the pharmacokinetics of valproic acid and quetiapine when divalproex sodium (sodium valproate and valproic acid in a 1:1 molar ratio) and Quetiapine were co-administered.
Medicines that depress the central nervous system and ethanol increase the risk of side effects.
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25 ° C.
Shelf life
Shelf life
2 years.
Manufacturer
Manufacturer
Vertex, Russia
Additional information
| Shelf life | 2 years. |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature not exceeding 25 ° C. |
| Manufacturer | Vertex, Russia |
| Medication form | pills |
| Brand | Vertex |
Other forms…
Related products
Buy Quetiapine, 100 mg 60 pcs with delivery to USA, UK, Europe and over 120 other countries.