No products in the cart.
Polyoxidonium, lyophilizate 6 mg 5 pcs
€40.36 €33.63
Description
Pharmacotherapeutic group:
immunomodulatory agent
ATC code: [L0Z]
Pharmacological ACTION
Polioxidonium® has an immunomodulatory effect and increases the body’s resistance to local and generalized infections. The basis of the mechanism of immunomodulatory action of Polioxidonium® is a direct effect on phagocytic cells and natural killers and stimulation of antibody formation.
Polioxidonium® restores immunity in cases of secondary immunodeficiency conditions caused by various infections, injuries, burns, autoimmune diseases, malignant tumors, complications of surgery, chemotherapeutic agents, cytostatics, steroid hormones.
In addition to its immunomodulating action, Polioxidonium® has strong detoxifying and antioxidant activity, detoxifying the body to remove toxins and salts of heavy metals, and inhibits lipid peroxidation. These properties are determined by the structure and the high-molecular nature of Polioxidonium®.
Inclusion of this medicine in the treatment of cancer patients reduces intoxication because of chemotherapy and radiation therapy and in most cases allows to perform standard therapy without changing the regimen due to the development of infectious complications and side effects (myelosuppression, vomiting, diarrhea, cystitis, colitis etc.).
The use of Polioxidonium® against the background of secondary immunodeficiency states can improve the effectiveness and shorten the duration of treatment, significantly reduce the use of antibiotics, bronchodilators, glucocorticosteroids and extend the period of remission.
The drug is well tolerated, has no mitogenic, polyclonal activity, antigenic properties and has no allergic, mutagenic, embryotoxic, teratogenic and carcinogenic action.
Pharmacokinetics
Polioxidonium® by intramuscular administration has high bioavailability (89 %); time of maximum concentration in blood – 40 minutes; fast distribution in all organs and tissues. Period of half-distribution in an organism (fast phase) – 0.44 hours, period of half-excretion (slow phase) – 36.2 hours. In the body the drug is hydrolyzed to oligomers, which are excreted mainly by the kidneys.
Indications
Indications
Correction of immunity in adults and children from 6 months.
In adults in complex therapy:
• chronic recurrent infectious and inflammatory diseases that are not amenable to standard therapy in the acute stage and in remission;
• acute and chronic viral and bacterial infections (including urogenital infectious and inflammatory diseases);
• tuberculosis;
• acute and chronic allergic diseases (including hay fever, bronchial asthma, atopic dermatitis), complicated by chronic recurrent bacterial and viral infections;
• in oncology during and after chemotherapy and radiation therapy to reduce the immunosuppressive, nephro- and hepatotoxic effects of drugs;
• to activate regenerative processes (fractures, burns, trophic ulcers);
• rheumatoid arthritis treated for a long time with immunosuppressants; with rheumatoid arthritis complicated by acute respiratory infections;
• for the prevention of postoperative infectious complications;
• for the prevention of influenza and acute respiratory infections
In children in complex therapy:
• acute and chronic inflammatory diseases caused by pathogens of bacterial, viral, fungal infections (including ENT organs – sinusitis, rhinitis, adenoiditis, hypertrophy of the pharyngeal tonsil, ARVI);
• acute allergic and toxic-allergic conditions;
• bronchial asthma complicated by chronic respiratory tract infections;
• atopic dermatitis complicated by purulent infection;
• intestinal dysbiosis (in combination with specific therapy);
• for the rehabilitation of those who are often and long-term ill;
• prevention of influenza and acute respiratory infections.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group:
immunomodulatory agent
ATX code: [L0З]
PHARMACOLOGICAL ACTION
Polyoxidonium® has an immunomodulatory effect, increases the body’s resistance to local and generalized infections. The basis of the mechanism of immunomodulatory action of the drug Polyoxidonium® is a direct effect on phagocytic cells and natural killer cells, as well as stimulation of antibody formation.
Polyoxidonium® restores immunity in secondary immunodeficiency conditions caused by various infections, injuries, burns, autoimmune diseases, malignant neoplasms, complications after surgery, the use of chemotherapeutic agents, cytostatics, steroid hormones.
Along with the immunomodulatory effect, Polyoxidonium® has pronounced detoxification and antioxidant activity, has the ability to remove toxins and heavy metal salts from the body, and inhibits lipid peroxidation. These properties are determined by the structure and high-molecular nature of the drug Polyoxidonium®.
Its inclusion in the complex therapy of cancer patients reduces intoxication during chemotherapy and radiation therapy, and in most cases allows for standard therapy without changing the regimen due to the development of infectious complications and side effects (myelosuppression, vomiting, diarrhea, cystitis, colitis and others).
The use of the drug Polyoxidonium® against the background of secondary immunodeficiency states can increase the effectiveness and shorten the duration of treatment, significantly reduce the use of antibiotics, bronchodilators, glucocorticosteroids, and extend the period of remission.
The drug is well tolerated, does not have mitogenic, polyclonal activity, antigenic properties, does not have allergenic, mutagenic, embryotoxic, teratogenic and carcinogenic effects.
PHARMACOKINETICS
When administered intramuscularly, Polyoxidonium® has high bioavailability (89%); time to reach maximum concentration in the blood – 40 minutes; quickly distributed throughout all organs and tissues. The half-life of distribution in the body (fast phase) is 0.44 hours, the half-life (slow phase) is 36.2 hours. In the body, the drug is hydrolyzed to oligomers, which are excreted primarily by the kidneys.
Special instructions
Special instructions
If there is pain at the injection site, the drug is dissolved in 1 ml of 0.25% procaine solution if the patient does not have increased individual sensitivity to procaine.
Active ingredient
Active ingredient
Azoximer bromide
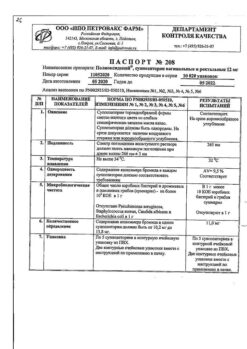
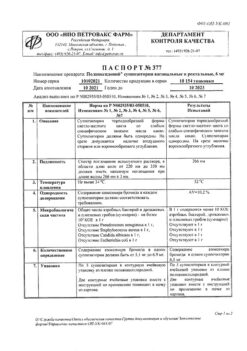
Composition
Composition
Composition for 1 ampoule or bottle:
Active substance:
Polyoxidonium® (Azoximer bromide) – 6 mg
Excipients:
mannitol,
povidone,
betacarotene – up to 9 mg.
Pregnancy
Pregnancy
Polyoxidonium is contraindicated during pregnancy, breastfeeding and children under 6 months (there is no clinical experience with its use).
Contraindications
Contraindications
Increased individual sensitivity. Pregnancy, lactation (no clinical experience of use).
WITH CAUTION
Acute renal failure, children under 6 months of age (clinical experience of use is limited).
Side Effects
Side Effects
Possible pain at the injection site when administered intramuscularly.
Interaction
Interaction
Polyoxidonium® is compatible with antibiotics, antiviral, antifungal and antihistamines, bronchodilators, glucocorticosteroids, and cytostatics.
Overdose
Overdose
Not described.
Storage conditions
Storage conditions
In a dry place at a temperature of 2 to 8 °C.
Keep out of the reach of children.
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
NPO Petrovax Pharm, Russia
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In a dry place at a temperature of 2 to 8 ° C. Store out of the reach of children. |
| Manufacturer | NPO Petrovax Pharm, Russia |
| Medication form | lyophilizate |
| Brand | NPO Petrovax Pharm |
Other forms…
Related products
Buy Polyoxidonium, lyophilizate 6 mg 5 pcs with delivery to USA, UK, Europe and over 120 other countries.