No products in the cart.
Piracetam, 200 mg capsules 60 pcs
€3.34 €2.97
Description
Pharmacotherapeutic group:
Notropic agent.
ATX code
[N06BX03].
Pharmacological properties
Pharmacodynamics
Piracetam is a cyclic derivative of gamma-aminobutyric acid (GABA), is a nootropic agent that directly affects the brain, improves cognitive processes (learning ability, memory, attention, mental performance). It affects the central nervous system in different ways: it changes the speed of excitation distribution in the brain, improves metabolic processes in nerve cells, improves microcirculation, affecting blood rheological characteristics and does not cause vasodilation.
Piracetam inhibits platelet aggregation and restores the elasticity of the red blood cell membrane, reduces red blood cell adhesion. At a dose of 9.6 g, it reduces the level of fibrinogen and Willibrand factors, prolongs the bleeding time.
It improves interhemispheric connections in the brain and synaptic conduction in the neocortical structures.
Piracetam has a protective and restorative effect in cases of brain dysfunction due to hypoxia and intoxication.
Limits the severity and duration of vestibular nystagmus.
Pharmacokinetics
In oral administration the drug is quickly and almost completely absorbed, the peak concentration is reached 1 hour after administration. The bioavailability of the drug is approximately 100%. After a single dose of 2 g the maximum concentration is 40-60 mcg/ml, which is reached in blood after 30 minutes and after 5 hours in cerebrospinal fluid after intravenous injection. Estimated volume of distribution of piracetam is about 0.6 l/kg. Period of half-life of the drug from plasma is 4-5 hours and 8.5 hours from cerebrospinal fluid, which is prolonged in case of renal insufficiency. Pharmacokinetics of piracetam does not change in patients with hepatic insufficiency.
It penetrates through the blood-brain and placental barrier and membranes used in hemodialysis. In animal studies piracetam selectively accumulates in tissues of the cerebral cortex, mainly in the frontal, parietal and occipital lobes, cerebellum and basal ganglia. It does not bind with blood plasma proteins and is not metabolized in the body. 80-100% of piracetam is excreted unchanged by the kidneys through renal filtration. Renal clearance of piracetam in healthy volunteers is 86 ml/min.
Indications
Indications
Symptomatic treatment of psychoorganic syndrome, in particular in elderly patients suffering from memory loss, dizziness, decreased concentration and general activity, mood changes, behavior disorder, gait disturbance, as well as in patients with Alzheimer’s disease and senile dementia of the Alzheimer’s type.
The consequences of ischemic stroke are speech disorders, emotional disturbances, decreased motor and mental activity.
Chronic alcoholism – for the treatment of psychoorganic and withdrawal syndromes.
The recovery period after brain injuries and intoxications.
Dizziness and related balance disorders, with the exception of dizziness of vasomotor and mental origin.
As part of complex therapy for learning disabilities in children with psychoorganic syndrome.
For the treatment of cortical myoclonus as mono- or complex therapy.
In the complex therapy of sickle cell anemia.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group:
nootropic agent.
ATX code
[N06BX03].
Pharmacological properties
Pharmacodynamics
Piracetam, a cyclic derivative of gamma-aminobutyric acid (GABA), is a nootropic drug that directly affects the brain, improves cognitive processes (learning ability, memory, attention, mental performance). It affects the central nervous system in various ways: it changes the speed of propagation of excitation in the brain, improves metabolic processes in nerve cells, improves microcirculation, affecting the rheological characteristics of the blood and does not cause vasodilation.
Piracetam inhibits platelet aggregation and restores the elasticity of the erythrocyte membrane, reduces the adhesion of erythrocytes. At a dose of 9.6 g, it reduces the level of fibrinogen and von Willibrand factors and prolongs bleeding time.
Improves interhemispheric connections in the brain and synaptic conduction in neocortical structures.
Piracetam has a protective and restorative effect in cases of impaired brain function due to hypoxia and intoxication.
Reduces the severity and duration of vestibular nystagmus.
Pharmacokinetics
When the drug is taken orally, it is quickly and almost completely absorbed, peak concentrations are reached 1 hour after administration. The bioavailability of the drug is approximately 100%. After taking a single dose of 2 g, the maximum concentration is 40-60 mcg/ml, which is achieved in the blood after 30 minutes and 5 hours in the cerebrospinal fluid after intravenous administration. The apparent volume of distribution of piracetam is about 0.6 l/kg. The half-life of the drug from blood plasma is 4-5 hours and 8.5 hours from cerebrospinal fluid, which is prolonged in case of renal failure. The pharmacokinetics of piracetam does not change in patients with liver failure.
Penetrates the blood-brain and placental barrier and membranes used in hemodialysis. In animal studies, piracetam selectively accumulates in the tissues of the cerebral cortex, mainly in the frontal, parietal and occipital lobes, cerebellum and basal ganglia. Does not bind to blood plasma proteins and is not metabolized in the body. 80-100% of piracetam is excreted unchanged by the kidneys by renal filtration. The renal clearance of piracetam in healthy volunteers is 86 ml/min.
Special instructions
Special instructions
When treating cortical myoclonus, abrupt interruption of treatment should be avoided, as this may cause resumption of attacks.
Penetrates through the filter membranes of hemodialysis machines.
Impact on the ability to drive vehicles and operate machinery
During the treatment period, care should be taken when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Piracetam
Composition
Composition
Active substance:
piracetam – 200 mg;
Excipients:
croscarmellose sodium – 6.0 mg,
magnesium stearate – 2.0 mg,
povidone-K17 – 5.0 mg;
Composition of the capsule body: titanium dioxide – 2.0%, gelatin up to 100%;
Composition of the capsule cap: black iron oxide dye – 0.53%, red iron oxide dye – 0.93%, yellow iron oxide dye – 0.2%, titanium dioxide – 0.3333%, gelatin up to 100%.
Pregnancy
Pregnancy
There have been no controlled studies of the use of the drug during pregnancy. Animal studies have not shown direct or indirect effects on pregnancy, embryo/fetal development, childbirth or postnatal development.
Piracetam penetrates the placental barrier and into breast milk. Plasma concentrations of piracetam in newborns reach 70-90% of those in the mother. Piracetam should not be prescribed during pregnancy.
During lactation, the issue of stopping breastfeeding should be decided.
Contraindications
Contraindications
Individual intolerance to piracetam or pyrrolidone derivatives, as well as other components of the drug.
Acute cerebrovascular accident (hemorrhagic stroke).
End-stage renal failure (with creatinine clearance less than 20 ml/min).
Children’s age up to 1 year.
Pregnancy and lactation.
Piracetam penetrates the placental barrier and into breast milk. The concentration of the drug in newborns reaches 70-90% of its concentration in the mother’s blood. Except in special circumstances, Piracetam should not be prescribed during pregnancy. You should refrain from breastfeeding when a woman is prescribed piracetam.
Side Effects
Side Effects
From the side of the central nervous system: motor disinhibition, irritability, drowsiness, depression, asthenia, headache, insomnia, mental agitation, imbalance, ataxia, exacerbation of epilepsy, anxiety, hallucinations, confusion.
From the digestive system: nausea, vomiting, diarrhea, abdominal pain.
Metabolism: increased body weight.
From the senses: vertigo.
From the skin: dermatitis, itching, urticaria.
Allergic reactions: hypersensitivity, anaphylactic reactions, angioedema.
Local reactions: pain at the injection site, thrombophlebitis
Other (with parenteral administration): fever, decreased blood pressure.
Interaction
Interaction
The possibility of changing the pharmacokinetics of piracetam under the influence of other drugs is low, because 90% of piracetam is excreted unchanged by the kidneys.
When used concomitantly with thyroid hormones, there have been reports of confusion, irritability, and sleep disturbances.
According to a published study in patients with recurrent venous thrombosis, piracetam at a dose of 9.6 g/day increases the effectiveness of indirect anticoagulants (there was a more pronounced decrease in platelet aggregation, fibrinogen levels, von Willebrand factors, blood and plasma viscosity compared with the use of indirect anticoagulants only).
In vitro, piracetam does not inhibit cytochrome P450 isoenzymes, such as CYP1A2, 2B6, 2C8, 2C9, 2C19, 2B6, 2E1 and 4A9/11 at concentrations of 142, 426 and 1422 μg/ml. At a concentration of 1422 mcg/ml, a slight inhibition of CYP2A6 (21%) and ZA4/5 (11%) was noted, however, the Ki level of these two isoenzymes is sufficient when exceeding 1422 mcg/ml, and therefore metabolic interaction with other drugs is unlikely.
Taking piracetam at a dose of 20 g/day for 4 weeks did not change the maximum serum concentration and the area under the concentration-time curve of antiepileptic drugs (carbamazepine, phenytoin, phenobarbital, valproic acid).
Co-administration with alcohol did not affect serum concentrations of piracetam; The concentration of ethanol in the blood serum did not change when taking 1.6 g of piracetam.
Overdose
Overdose
Symptoms: diarrhea mixed with blood, abdominal pain.
Treatment: in case of significant overdose, rinse the stomach or induce vomiting. Symptomatic therapy is recommended, which may include hemodialysis. There is no specific antidote. The effectiveness of hemodialysis for piracetam is 50-60%.
Manufacturer
Manufacturer
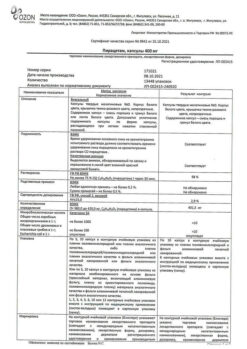
Ozon, Russia
Additional information
| Manufacturer | Ozon, Russia |
|---|---|
| Medication form | capsules |
| Brand | Ozon |
Other forms…
Related products
Buy Piracetam, 200 mg capsules 60 pcs with delivery to USA, UK, Europe and over 120 other countries.