Subtotal: €3.24
Otrivin Complex, spray 10 ml
€10.01 €8.34
Xylometazoline belongs to the group of local vasoconstrictors (decongestants) with alpha-adrenomimetic effect, causes narrowing of blood vessels of the nasal mucosa, thereby eliminating edema and hyperemia of the nasopharyngeal mucosa. It relieves congestion making nasal breathing easier in rhinitis.
Ipratropium bromide has anticholinergic effect. When administered intranasally it decreases nasal secretion stopping nasal flow due to competitive inhibition of cholinergic receptors located in the epithelium of the nasal cavity.
In therapeutic concentrations it does not irritate the mucous membrane, does not cause hyperemia.
The drug starts working in 5-10 minutes and has a sustained effect for 6-8 hours.
Indications
Symptomatic treatment of edema and hyperemia of the nasal cavity, acute respiratory diseases with symptoms of rhinitis (runny nose), accompanied by congestion, acute allergic rhinitis, pollinosis, sinusitis.
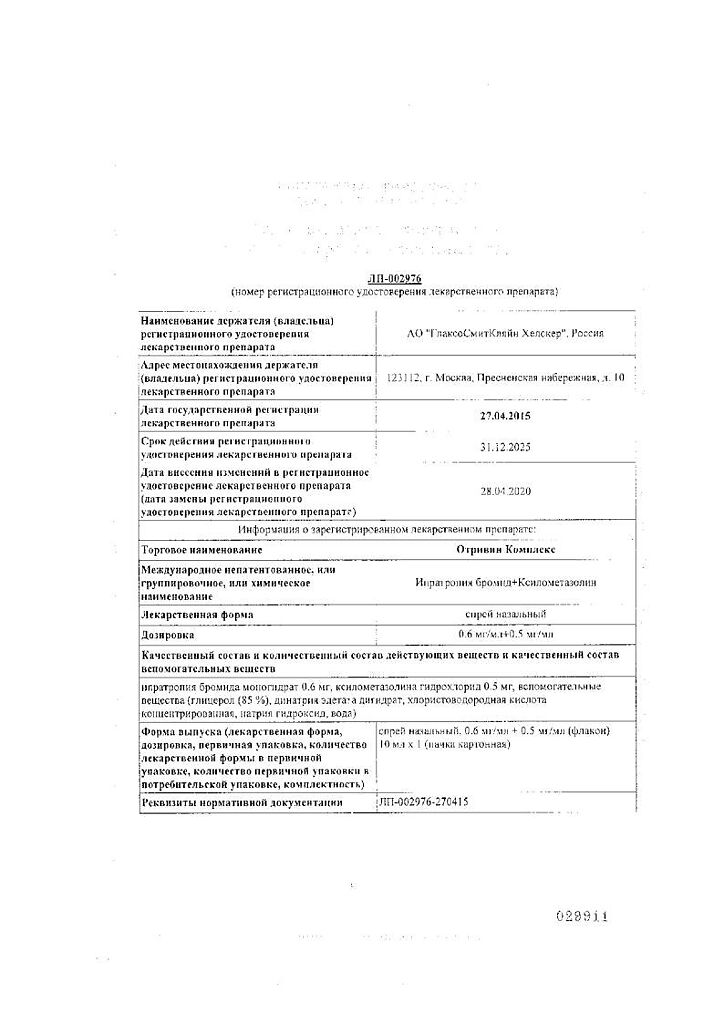
Active ingredient
Composition
Active ingredients:
Ipratropium bromide monohydrate 0.6 mg,
xylometazoline hydrochloride 0.5 mg;
Supplements:
Glycerol (85%) 27.9 mg,
disodium edetate dihydrate 0.5 mg,
Hydrochloric acid concentrated to pH 4.5,
sodium hydroxide to pH 4.5,
water to 1 ml.
How to take, the dosage
Intranasally.
For patients over 18 years of age: 1 injection into each nasal passage 3 times a day. The drug should not be used for more than 7 days without medical advice. Prolonged use of xylometazoline may cause nasal mucosal edema and increased secretion due to development of the cells’ hypersensitivity to the active substances of the drug, the so-called reverse effect.
One Injection of Otrivin® Complex contains approximately 70 micrograms of xylometazoline hydrochloride and 84 micrograms of ipratropium bromide.
Interaction
The drug is incompatible with monoamine oxidase inhibitors, tri- and tetracyclic antidepressants.
Sympathomimetic drugs cause release of catecholamines, including norepinephrine, which has a vasoconstrictor effect, resulting in increased blood pressure.
In case of significant increase in blood pressure the treatment with Otrivin® Complex should be stopped and symptomatic treatment provided.
Concomitant use with tri- and tetracyclic antidepressants may increase the sympathomimetic effect of xylometazoline.
In concomitant administration of other drugs with anticholinergic activity, the anticholinergic effect of ipratropium bromide may be enhanced.
Special Instructions
Nasal passages should be cleaned before use. Do not use for prolonged periods of time, e.g., if you have chronic rhinitis.
Should not get in or around the eyes. In case of contact, temporary blurred vision, irritation, pain, reddening of the eyes may occur, exacerbation of closed-angle glaucoma may develop. If the drug gets into the eyes, flush them thoroughly with cold water and consult a physician if you experience eye pain or blurred vision.
Impact on ability to drive vehicles and machines
The use of the drug Otrivin® Complex in therapeutic doses does not affect the ability to drive vehicles and machines.
Synopsis
Contraindications
Hypersensitivity to the components of the drug, hypersensitivity to atropine or similar compounds (hyoscyamine, scopolamine), glaucoma, brain surgery (history), pregnancy (first trimester), atrophic rhinitis, age under 18 years.
Side effects
Classification of the frequency of adverse reactions: Very common-more than 1/10 appointments (â¥10%); common-more than 1/100 but less than 1/10 appointments (â¥1% but â¤10%); infrequent-more than 1/1000 but less than 1/100 appointments (â¥0.1% but â¤1%); rare-more than 1/10000 but less than 1/1000 appointments (â¥0.01% but â¤0.1%); very rare-less than 1/10000 appointments (â¤0.01%).
Nervous system disorders:
Often: headache;
Rarely: dizziness;
Very rarely: insomnia.
Digestive system disorders:
Often: dry mouth;
Infrequent: dyspepsia, nausea.
Senses:
Rarely: increased intraocular pressure, mydriasis, eye pain, worsening in closed-angle glaucoma;
Very rarely: impaired visual clarity. Urinary system disorders:
Rarely: difficulty urinating.
Respiratory system disorders:
Often: nasal bleeding, irritation and/or dryness of nasopharyngeal mucosa, burning sensation, tingling, sneezing, nasal hypersecretion;
Infrequently: nasal congestion (with frequent and/or prolonged use of the drug).
Cardiovascular system disorders:
Rarely: palpitations, supraventricular tachycardia, atrial fibrillation;
Very rarely: arrhythmic pulse.
Skin disorders:
Rarely: itching, skin rash, urticaria.
Allergic reactions:
Rarely: systemic allergic reactions (anaphylactic reaction, angioedema of the tongue, lips and face, laryngospasm).
If any of the side effects listed in the instructions worsen, or if you notice any other side effects not listed in the instructions, tell your doctor.
Overdose
In nasal administration of Otrivin® Complex acute overdose is unlikely, since the absorption of the drug is extremely low.
In case of xylomethasoline overdose the clinical picture is characterized by: nausea, sweating, decreased body temperature, headache, bradycardia, accommodation disorder, arterial hypertension, respiratory depression, coma. Hypertension may be replaced by hypotension. Symptomatic therapy should be carried out under the supervision of a physician.
In overuse of ipratropium bromide overdose is unlikely due to extremely low absorption of the substance into the blood, but dry mouth, difficulty of accommodation, tachycardia may develop. Treatment is symptomatic.
Significant overdose may cause CNS symptoms associated with the cholinolytic effect of the drug, including hallucinations for which cholinesterase inhibitors are prescribed.
Pregnancy use
Similarities
| Weight | 0.030 kg |
|---|---|
| Shelf life | 3 years. |
| Conditions of storage | The drug should be kept out of reach of children at a temperature not exceeding 25 ° C. |
| Manufacturer | GSC Consumer Healthcare S.A., Switzerland |
| Medication form | nasal spray |
| Brand | GSC Consumer Healthcare S.A. |
Related products
Buy Otrivin Complex, spray 10 ml with delivery to USA, UK, Europe and over 120 other countries.