No products in the cart.
Orvis Rhino, drops 100 ml
€1.00
Out of stock
(E-mail when Stock is available)
Description
A combined preparation of herbal origin.
The pharmacological properties are due to the biologically active substances included in the drug.
The ORVIS® Rhino has secretolytic, secretomotor, anti-inflammatory, anti-edema, moderate antibacterial, antiviral effect. It promotes the outflow of exudate from the sinuses and upper respiratory tracts, preventing the development of complications.
Indications
Indications
Acute and chronic sinusitis, accompanied by the formation of viscous secretion
Pharmacological effect
Pharmacological effect
Combined preparation of plant origin.
Pharmacological properties are determined by the biologically active substances that make up the drug.
ORVIS® Rhino has secretolytic, secretomotor, anti-inflammatory, decongestant, moderate antibacterial, antiviral effects. Promotes the outflow of exudate from the paranasal sinuses and upper respiratory tract, preventing the development of complications.
Special instructions
Special instructions
The drug contains 16.0-19.5% ethanol (volume).
A single dose of the drug for children from 2 to 6 years (15 drops) contains up to 0.14 g of absolute ethanol (ethyl alcohol); a single dose of the drug for school-age children (25 drops) contains up to 0.24 g of absolute ethanol (ethyl alcohol); a single dose of the drug for adults (50 drops) contains up to 0.47 g of absolute ethanol (ethyl alcohol).
The daily dose of the drug for adults (150 drops) contains up to 1.42 g of absolute ethanol (ethyl alcohol).
When using, the bottle should be kept in an upright position.
During storage of ORVIS® Rhino drops, slight turbidity or slight precipitation may occur, which does not affect the effectiveness of the drug.
Shake before use!
Impact on the ability to drive vehicles. Wed and fur.:
During the period of use of the drug, care must be taken when performing potentially hazardous activities that require increased concentration of attention and speed of psychomotor reactions (driving vehicles, working with moving mechanisms, working as a dispatcher, operator) due to the content of 16.0-19.5% ethanol in the drug (volume ratio).
Active ingredient
Active ingredient
Plant extracts
Composition
Composition
100 g drops contain
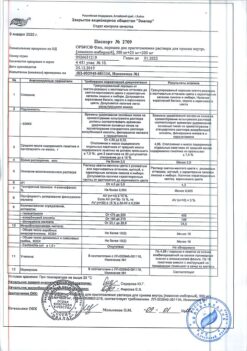
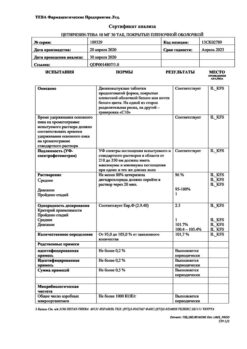
Active components: 29 g of aqueous-alcoholic extract from medicinal plant raw materials: yellow gentian roots – 0.2 g, black elderberry flowers – 0.6 g, verbena herb – 0.6 g, spring primrose flowers – 0.6 g, sorrel herb – 0.6 g.
Excipients: purified water – 71.0 g.
Ethanol content (ethyl alcohol): 16.0 – 19.5% (volume).
Yellow gentian – Gentiana lutea L., Gentian family (Gentianaceae), black elderberry – Sambucus nigra L., Elder family (Sambucaceae) (Honeywort family (Caprifoliaceae)), verbena – Verbena officinalis L., Verbenaceae family (Verbenaceae), spring primrose – Primula veris L., family Primroses (Primulaceae), sour sorrel – Rumex acetosa L., Buckwheat family (Polygonaceae).
Pregnancy
Pregnancy
The use of ORVIS® Rhino during pregnancy is possible only as prescribed by a doctor if the benefits of use outweigh the potential risk to the fetus.
The drug is not recommended for use during breastfeeding (due to the lack of experience in its clinical use).
Contraindications
Contraindications
Hypersensitivity to the components of the drug, alcoholism, children (up to 2 years).
Patients should not take the drug after successful anti-alcohol treatment due to the ethanol (ethyl alcohol) content.
With caution:
The use of the drug in patients with liver diseases, epilepsy, diseases and brain injuries is possible only after consultation with a doctor (due to the content of ethanol (ethyl alcohol)).
Side Effects
Side Effects
Allergic reactions are possible (skin rash, redness of the skin, itching, angioedema (Quincke’s edema), shortness of breath), gastrointestinal disorders (pain in the epigastric region, nausea).
If side effects occur, you should stop taking the drug and consult a doctor.
Interaction
Interaction
Combination with antibacterial drugs is possible and advisable.
Interaction with other drugs is not known to date.
Overdose
Overdose
In case of overdose, the severity of dose-dependent side effects may increase.
Treatment is symptomatic.
In case of overdose of the drug, you should immediately consult a doctor.
Recommendations for use
Recommendations for use
Inside, after diluting it in a small amount of water.
Adults: 50 drops 3 times a day.
School-age children: 25 drops 3 times a day.
Children from 2 to 6 years: 15 drops 3 times a day.
The course of treatment is 7-14 days.
The drug should be taken after meals.
If symptoms persist for more than 7-14 days or recur periodically, you should consult your doctor.
Manufacturer
Manufacturer
Evalar CJSC, Russia
Additional information
| Manufacturer | Evalar, Russia |
|---|---|
| Medication form | oral drops |
| Brand | Evalar |
Other forms…
Related products
Buy Orvis Rhino, drops 100 ml with delivery to USA, UK, Europe and over 120 other countries.