No products in the cart.
Nazarel, spray 50 mcg/dose 120 doses
€13.83 €11.98
Description
Pharmacotherapeutic group: glucocorticosteroid for topical use
ATC code: R01AD08
Pharmacological properties
Pharmacodynamics
Mechanism of action. Fluticasone propionate is a substance with strong anti-inflammatory action. When administered intranasally no marked systemic action and suppression of hypothalamic-pituitary-adrenal system is noted.
No significant changes of the daily area under the pharmacokinetic curve of serum cortisol after administration of fluticasone propionate at a dose of 200 mcg/day compared to placebo (ratio: 1.01, 90% CI – confidence interval 0.9 to 1.14).
Fluticasone propionate has anti-inflammatory effect due to its interaction with glucocorticosteroid receptors. It suppresses proliferation of mast cells, eosinophils, lymphocytes, macrophages, neutrophils, reduces the production of inflammatory mediators and other biologically active substances (histamine, prostaglandins, leukotrienes, cytokines) during the early and late phase of the allergic reaction. Fluticasone propionate has a rapid anti-inflammatory effect on the nasal mucosa, and its anti-allergic effect is seen as early as 2-4 hours after the first use. It relieves sneezing, nasal itching, runny nose, nasal congestion, nasal sinus discomfort and feeling of pressure around the nose and eyes. Also relieves eye symptoms associated with allergic rhinitis. Reduction of symptoms (especially nasal congestion) persists for 24 hours after a single administration of 200 mcg spray. Fluticasone propionate improves patients’ quality of life, including physical and social activity.
Pharmacokinetics
Intake. After intranasal administration of fluticasone propionate at a dose of 200 mcg/day, maximum equilibrium plasma concentrations are not quantifiable in most patients (less than 0.01 ng/ml). The highest plasma concentration was recorded at 0.017 ng/ml. Direct nasal absorption is unlikely due to low water solubility and swallowing most of the drug. Absolute oral bioavailability is low (less than 1%) due to a combination of incomplete absorption from the gastrointestinal tract and active metabolism during first passage through the liver. Total systemic absorption is thus extremely low.
Distribution. Fluticasone propionate has a large volume of distribution in the equilibrium state (approximately 318 l). The binding to plasma proteins is high (91%).
Metabolism. Fluticasone propionate is rapidly eliminated from the systemic bloodstream primarily by metabolism in the liver to form inactive carboxylic acid via CYP3A4 isoenzyme of cytochrome P450 system. Metabolism of the swallowed fraction of fluticasone propionate on first passage through the liver occurs in the same manner.
Excretion. Excretion of fluticasone propionate is linear in the dose range from 250 to 1000 µg and is characterized by high plasma clearance (1.1 L/min). Maximum plasma concentrations are reduced by approximately 98% within 3-4 hours, and only at very low plasma concentrations was a final half-life of 7.8 hours observed. Renal clearance of fluticasone propionate is insignificant (less than 0.2%) and of the inactive metabolite, carboxylic acid, less than 5%. Fluticasone propionate and its metabolites are mainly excreted with the bile through the intestine.
Indications
Indications
Treatment of year-round and seasonal allergic rhinitis, including hay fever (hay fever) in adults and children over 4 years of age (elimination of symptoms, such as pain and pressure in the paranasal sinuses, nasal congestion, runny nose, sneezing, itchy nose, lacrimation).
If there is no improvement or you feel worse after 4 days, you should consult a doctor.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: glucocorticosteroid for local use
ATX code: R01AD08
Pharmacological properties
Pharmacodynamics
Mechanism of action. Fluticasone propionate is a substance with a strong anti-inflammatory effect. With intranasal administration, no pronounced systemic effect or inhibition of the hypothalamic-pituitary-adrenal system is observed.
There was no significant change in the daily area under the pharmacokinetic curve of serum cortisol after administration of fluticasone propionate at a dose of 200 mcg/day compared with placebo (ratio: 1.01, 90% CI – confidence interval 0.9 to 1.14).
Fluticasone propionate has an anti-inflammatory effect due to interaction with glucocorticosteroid receptors. Suppresses the proliferation of mast cells, eosinophils, lymphocytes, macrophages, neutrophils; reduces the production of inflammatory mediators and other biologically active substances (histamine, prostaglandins, leukotrienes, cytokines) during the early and late phases of the allergic reaction. Fluticasone propionate has a rapid anti-inflammatory effect on the nasal mucosa, and its antiallergic effect appears within 2-4 hours after the first use. Reduces sneezing, itchy nose, runny nose, nasal congestion, discomfort in the sinuses and pressure around the nose and eyes. In addition, it relieves eye symptoms associated with allergic rhinitis. Reduction in the severity of symptoms (especially nasal congestion) persists for 24 hours after a single administration of a spray at a dose of 200 mcg. Fluticasone propionate improves the quality of life of patients, including physical and social activity.
Pharmacokinetics
Suction. After intranasal administration of fluticasone propionate at a dose of 200 mcg/day, maximum steady-state plasma concentrations are not quantitatively determined in most patients (less than 0.01 ng/ml). The highest plasma concentration was recorded at 0.017 ng/mL. Direct absorption from the nasal cavity is unlikely due to low water solubility and ingestion of most of the drug. Absolute oral bioavailability is low (less than 1%) as a result of a combination of incomplete absorption from the gastrointestinal tract and extensive first-pass metabolism through the liver. The total systemic absorption is therefore extremely low.
Distribution. Fluticasone propionate has a large volume of distribution at steady state (approximately 318 L). High binding to plasma proteins (91%).
Metabolism. Fluticasone propionate is rapidly eliminated from the systemic circulation mainly due to metabolism in the liver with the formation of inactive carboxylic acid via the CYP3A4 isoenzyme of the cytochrome P450 system. The metabolism of the ingested fraction of fluticasone propionate during the first passage through the liver occurs in the same way.
Excretion. The elimination of fluticasone propionate is linear in the dose range from 250 to 1000 mcg and is characterized by a high plasma clearance (1.1 l/min). The maximum plasma concentration is reduced by approximately 98% within 3-4 hours, and only at very low plasma concentrations was a terminal half-life of 7.8 hours observed. The renal clearance of fluticasone propionate is insignificant (less than 0.2%), and the inactive metabolite, carboxylic acid, is less than 5%. Fluticasone propionate and its metabolites are mainly excreted in bile through the intestines.
Special instructions
Special instructions
Driving vehicles and working with machinery
Given the possibility of developing undesirable reactions, care must be taken when driving vehicles and working with machinery. Be sure to consult your doctor.
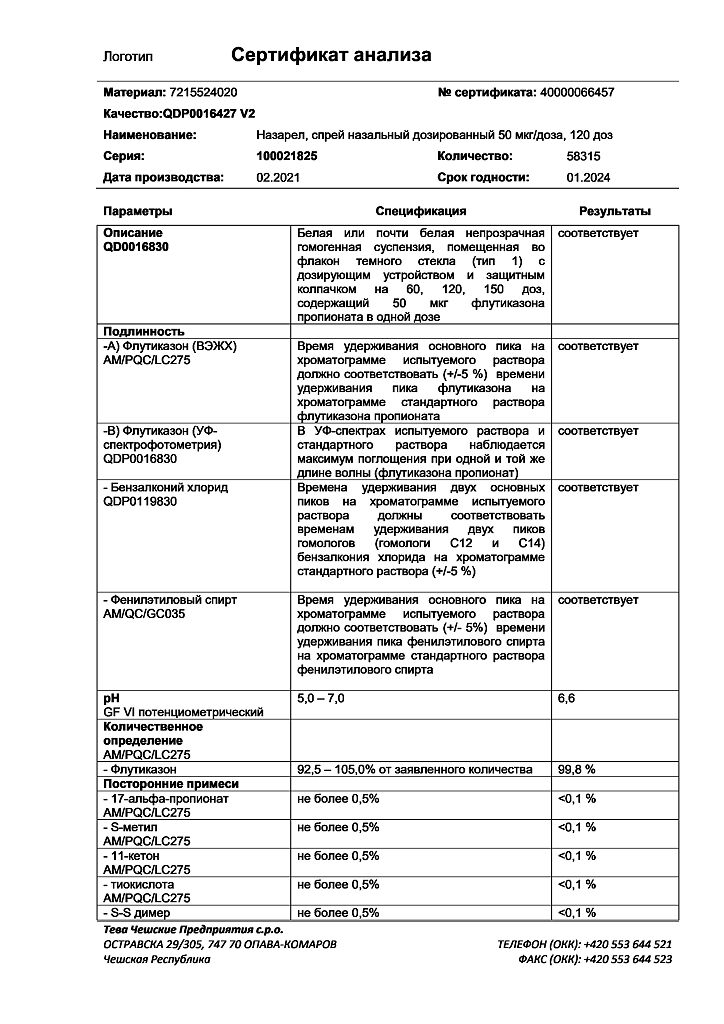
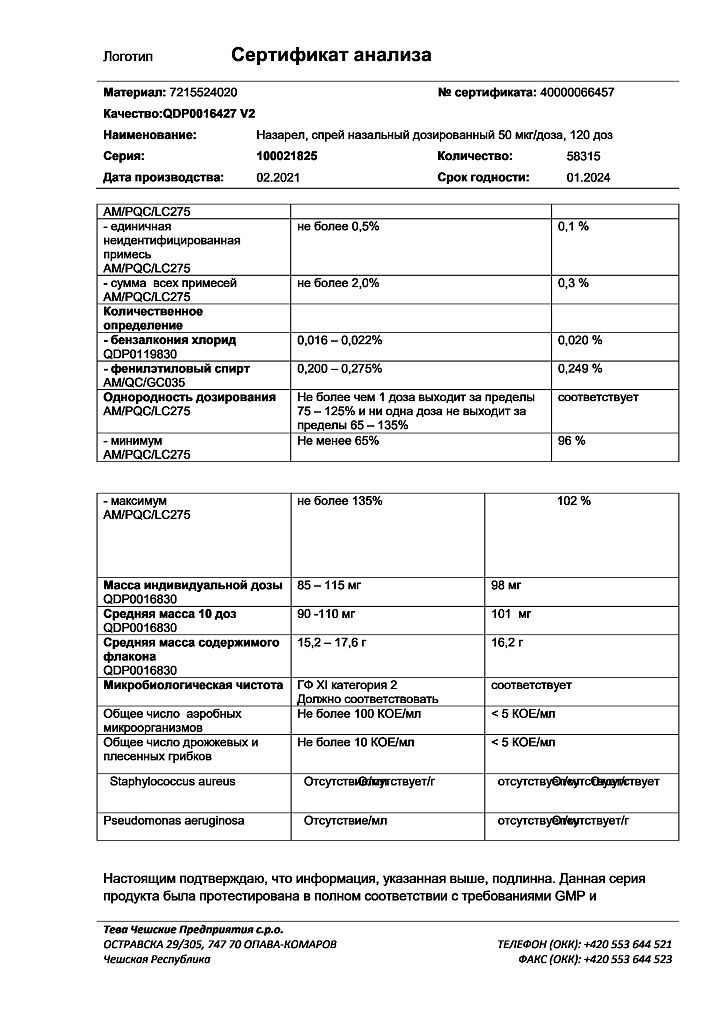
The drug Nazarel contains benzalkonium chloride.
Benzalkonium chloride may cause irritation and swelling of the nasal mucosa.
Active ingredient
Active ingredient
Fluticasone
Composition
Composition
The active ingredient is fluticasone.
Each dose contains 50 mcg of fluticasone (as propionate).
Excipients are polysorbate 80, microcrystalline cellulose and carmellose sodium, anhydrous glucose, benzalkonium chloride (50% solution), phenylethanol, purified water.
Pregnancy
Pregnancy
If you are pregnant or breastfeeding, think you may be pregnant, or are planning a pregnancy, consult your doctor or pharmacist before using this medicine.
Contraindications
Contraindications
Do not use Nazarel:
if you are allergic to fluticasone or any of the other ingredients of this medicine listed in section 6 of the leaflet;
if you have recently had a nasal injury or surgery in the nasal cavity.
Special instructions and precautions
Before using Nazarel, consult your doctor or pharmacist.
The drug is indicated for intranasal use only.
To achieve maximum therapeutic effect, adhere to a regular dosage regimen.
If there is no improvement or you feel worse after 4 days, consult your doctor.
Do not use Nazarel for more than 3 months in adults and children over 12 years of age. If you or your child need to use the drug for more than 3 months, consult your doctor.
Do not use Nazarel for more than 2 months in children aged 4 to 12 years.
If your child needs to use the drug for more than 2 months, you should consult your doctor. With long-term use, the doctor may decide to regularly monitor the function of the adrenal cortex.
When using nasal glucocorticosteroids, systemic effects may occur, especially when used in high doses for a long time. These effects are much less likely to occur than with oral corticosteroids and may vary in individual patients and between different corticosteroid medications.
Possible systemic effects may include Cushing’s syndrome, Cushing’s signature features, adrenal suppression, growth retardation in children and adolescents, clouding of the lens of the eye (cataract), eye disease that causes visual impairment (glaucoma), and less commonly a number of psychological or behavioral effects, including psychomotor hyperactivity, sleep disturbances, anxiety, depression, or aggression (especially in children).
Talk to your doctor if you or your child are taking drugs to treat HIV (such as ritonavir).
If you or your child develop new symptoms, such as severe facial pain or thick nasal discharge, which may indicate an infection and may not be related to an allergy, contact your doctor.
The drug Nazarel eliminates the symptoms of seasonal allergic rhinitis, however, in some cases, with very high concentrations of allergens in the air, to relieve eye symptoms, for example, severe itching in the eyes, consult your doctor for additional therapy.
Tell your doctor if you or your child have recently received injectable glucocorticosteroids or taken glucocorticosteroids by mouth for a long time.
If you or your child have been in contact with people with chickenpox, measles, or if there are changes in your vision, stop treatment and contact your doctor.
Children
Do not use the drug in children aged 0 to 4 years due to the risk of ineffectiveness and possible unsafety (the safety and effectiveness of the drug in children under 4 years of age have not been established).
Children receiving therapy with certain intranasal glucocorticosteroids in approved doses may experience a decrease in growth rate. It is recommended to regularly monitor the growth of children receiving long-term treatment with intranasal glucocorticosteroids. If your child’s growth slows, contact your doctor.
Side Effects
Side Effects
Like all medicines, this medicine can cause side effects, although not everyone gets them.
Stop using Nazarel and get medical help right away if any of the following signs of an allergic reaction occur, which are very rare (may occur in up to 1 in 10,000 people):
bronchospasm;
skin rash;
swelling of the face and tongue;
severe allergic reactions, usually occurring after repeated contact with the allergen (anaphylactic reactions);
severe allergic reactions, usually occurring after the first contact with an allergen (anaphylactoid reactions).
Other possible adverse reactions that may occur while taking Nazarel
Very common (may affect more than 1 in 10 people):
nosebleed.
Common (may affect up to 1 in 10 people):
headache;
sensation of unpleasant taste and smell;
dryness of the mucous membrane in the nasal cavity and pharynx;
irritation of the mucous membrane in the nasal cavity and pharynx.
Very rare (may affect up to 1 in 10,000 people):
eye disease leading to visual impairment (glaucoma);
increased intraocular pressure;
clouding of the lens of the eye (cataract);
a through defect (hole) of the nasal septum (perforation of the nasal septum) (also reported when taking other intranasal glucocorticosteroids);
ulcers in the nose.
Unknown (frequency cannot be determined based on available data):
blurred vision.
When using some intranasal glucocorticosteroids, systemic effects may develop, especially when prescribed in high doses for a long period of time.
Interaction
Interaction
Tell your doctor or pharmacist if you are taking, have recently taken, or may start taking any other medications.
In particular, tell your doctor if you are taking any of the following drugs:
– Medicines used to treat fungal infections (for example, ketoconazole and itraconazole);
– Some drugs may increase the effects of Nazarel, and your doctor may decide that you need to be monitored closely if you take these drugs (including some HIV drugs, such as ritonavir and cobicistat).
Overdose
Overdose
There is no evidence of acute or chronic overdose of fluticasone propionate.
In healthy volunteers, intranasal administration of 2 mg of fluticasone propionate twice daily for 7 days had no effect on the function of the hypothalamic-pituitary-adrenal axis (doses 20 times higher than the therapeutic dose). Use of the drug in doses higher than recommended for a long period of time may lead to temporary suppression of adrenal function.
In case of overdose, consult a doctor.
Storage conditions
Storage conditions
Keep the drug out of the reach of children and so that the child cannot see it. Do not use the drug after the expiration date (shelf life) indicated on the bottle or carton after “Best before:”. The expiration date is the last day of the given month.
Store the drug at a temperature not exceeding 25 °C in the original packaging (bottle in a pack).
After opening the bottle, the drug is suitable for use for 3 months.
Do not throw away (pour) the drug into the sewer or water supply.
Ask your pharmacist how to dispose of (destroy) a drug that is no longer needed. These measures will protect the environment.
Manufacturer
Manufacturer
Teva Czech Enterprises s.r.o., Czech Republic
Additional information
| Shelf life | 3 years. Do not use after the expiration date. |
|---|---|
| Conditions of storage | Store at a temperature not exceeding 25° C. Keep out of reach of children! |
| Manufacturer | Teva Czech Enterprises s.r.o., Czech Republic |
| Medication form | nasal spray |
| Brand | Teva Czech Enterprises s.r.o. |
Related products
Buy Nazarel, spray 50 mcg/dose 120 doses with delivery to USA, UK, Europe and over 120 other countries.