No products in the cart.
Memoplant, 120 mg 30 pcs
€36.28 €30.23
Description
Pharmacotherapeutic group
An angioprotective agent of plant origin.
ATX code
N06DX02
/p>
Pharmacological properties
Pharmacodynamics
It increases the body’s resistance to hypoxia, especially brain tissue.
Enhances cerebral and peripheral blood circulation, improves blood rheology. It has a dose-dependent regulatory effect on the vascular wall, dilates small arteries and increases the tone of veins. Prevents the formation of free radicals and lipid peroxidation of cell membranes. It improves metabolism in organs and tissues, promotes the accumulation of macroergies in cells, increases the utilization of oxygen and glucose, and normalizes mediator processes in the central nervous system.
Pharmacokinetics
Assimilation
After oral administration, the bioavailability of the terpenlactones (ginkgolide A, ginkgolide B, and bilobalide) is 80% for ginkgolide A, 88% for ginkgolide B, and 79% for bilobalide.
Distribution
. Peak plasma concentrations were 16-22 ng/mL for ginkgolide A, 8- 10 ng/mL for ginkgolide B, and 27-54 ng/mL for bilobalide after administration in tablet form. Binding to plasma proteins is: 43% for ginkgolide A, 47% for ginkgolide B, and 67% for bilobalide.
Evolution
The elimination half-life is 3.9 hours (ginkgolide A), 4-6 hours (ginkgolide B) and 2-3 hours (bilobalide).
Indications
Indications
• Brain dysfunctions, including age-related ones, associated with cerebrovascular accidents, accompanied by the following symptoms: memory impairment, decreased ability to concentrate and intellectual abilities, dizziness, tinnitus, headache.
• Peripheral circulatory disorders: obliterating diseases of the arteries of the lower extremities with such characteristic symptoms as intermittent claudication, numbness and coldness of the feet, Raynaud’s disease.
• Disorders of the inner ear, manifested by dizziness, unsteady gait, tinnitus.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group
Angioprotective agent of plant origin.
ATX code
N06DX02
Pharmacological properties
Pharmacodynamics
Increases the body’s resistance to hypoxia, especially brain tissue.
Improves cerebral and peripheral blood circulation, improves blood rheology. It has a dose-dependent regulatory effect on the vascular wall, dilates small arteries, and increases the tone of the veins. Prevents the formation of free radicals and lipid peroxidation of cell membranes. Improves metabolism in organs and tissues, promotes the accumulation of macroergs in cells, increases the utilization of oxygen and glucose, and normalizes mediator processes in the central nervous system.
Pharmacokinetics
Suction
After oral administration, the bioavailability of the terpene lactones (ginkgolide A, ginkgolide B and bilobalide) is 80% for ginkgolide A, 88% for ginkgolide B and 79% for bilobalide.
Distribution
Peak plasma concentrations were 16-22 ng/ml for ginkgolide A, 8-10 ng/ml for ginkgolide B and 27-54 ng/ml for bilobalide after administration in tablet form. Plasma protein binding is: 43% for ginkgolide A, 47% for ginkgolide B and 67% for bilobalide.
Removal
The half-life is 3.9 hours (ginkgolide A), 4-6 hours (ginkgolide B) and 2-3 hours (bilobalide).
Special instructions
Special instructions
Attention! All instructions must be strictly observed!
Before starting treatment with Ginkgo biloba extract EGb 761®, it is necessary to obtain advice on existing concomitant diseases and find out whether pathological symptoms are associated with these diseases.
The recommended dosage of the drug should not be exceeded. If a hypersensitivity reaction develops, the use of the drug should be discontinued. Before
surgical placement, you must inform your doctor about taking the drug. If you experience frequent feelings of dizziness and tinnitus, you should consult your doctor. In case of sudden deterioration or loss of hearing, you should immediately consult a doctor. Patients with bleeding (hemorrhagic diathesis) and patients receiving anticoagulant therapy should consult a doctor before starting drug therapy. While using the drug, patients suffering from epilepsy may experience epileptic seizures. Caution is recommended when co-administering drugs metabolized by cytochrome P450, including CYP3A4 (see section “Interaction with other drugs”).
Since there is evidence that drugs containing ginkgo biloba leaf extract can reduce blood clotting, the drug should be discontinued 3-4 days before surgery.
The drug contains lactose. Patients with hereditary lactose intolerance, lactase deficiency or glucose-galactose malabsorption should not take the drug.
Impact on the ability to drive vehicles and machinery
During the period of use of the drug, care should be taken when performing potentially hazardous activities that require increased concentration of attention and speed of psychomotor reactions (driving vehicles, working with moving mechanisms, working as a dispatcher, operator).
Active ingredient
Active ingredient
Ginkgo biloba leaf extract
Composition
Composition
(for 1 tablet)
Active ingredient:
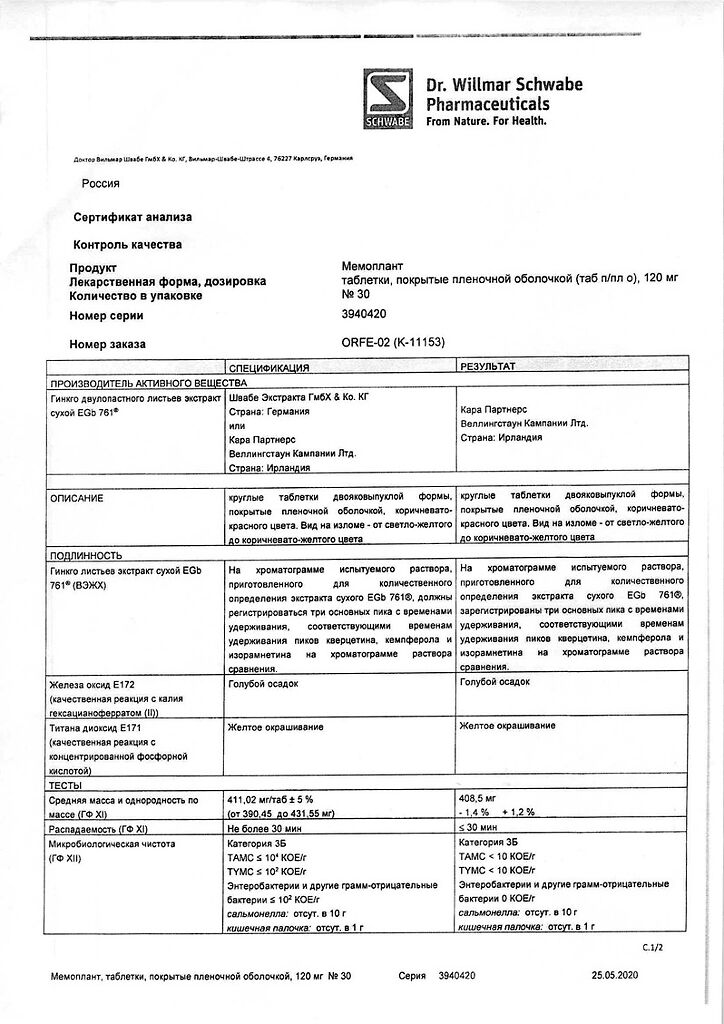
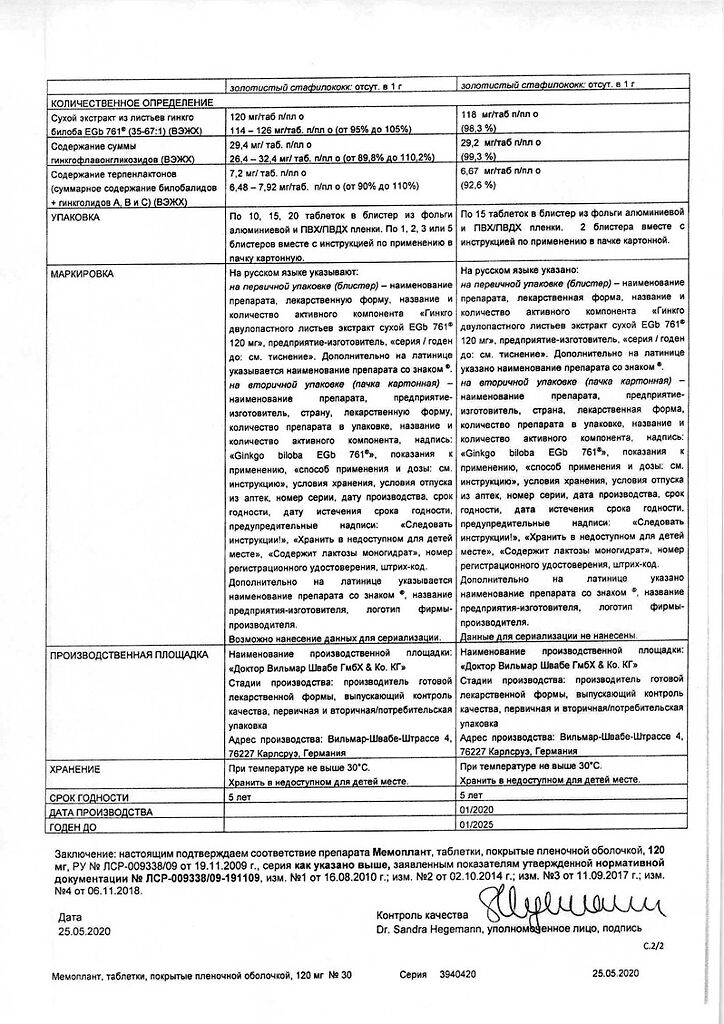
Ginkgo biloba leaf extract dry* EGb 761®** (35-67:1), extractant: acetone 60%, standardized for the content of ginkgoflavone glycosides 29.4 mg and terpene lactones 7.2 mg (3.36-4.08 mg ginkgolides A, B, C and 3.12-3.84 mg bilobalides) 120.00 mg.
Auxiliary kernel components:
lactose monohydrate 68.25 mg; colloidal silicon dioxide 3.00 mg; microcrystalline cellulose 163.5 mg; corn starch 15.00 mg; croscarmellose sodium 15.00 mg; magnesium stearate 5.250 mg.
Additional shell components:
hypromellose 11.5728 mg; macrogol 1500 5.7812 mg; defoaming emulsion SE2 0.0150 mg ***; titanium dioxide (E 171) 1.6260 mg; iron oxide red (E 172) 1.3000 mg; talc 0.7200 mg.
*Dry extract obtained from leaves of Ginkgo biloba L., family: Ginkgoaceae
** Ginkgo biloba extract (manufacturer: Schwabe Extracta GmbH & Co.KG, Germany or Wallingstown Company Ltd. / Cara Partners, Ireland) EGb 761® (number assigned to the extract by the manufacturer).
***articles by Evr.F. on individual components of antifoam emulsion SE2
Pregnancy
Pregnancy
Due to the lack of sufficient clinical data, the use of the drug during pregnancy and breastfeeding is not recommended.
Contraindications
Contraindications
– Hypersensitivity to the active substances and/or to any of the excipients;
– decreased blood clotting;
– erosive gastritis in the acute stage;
– peptic ulcer of the stomach and duodenum in the acute stage;
– acute cerebrovascular accidents;
– acute myocardial infarction;
– age under 18 years (efficacy and safety have not been studied);
– lactose intolerance, lactase deficiency, glucose-galactose malabsorption.
With caution
Caution must be exercised when co-administering EGb761® and drugs metabolized by the CYP3A4 isoenzyme and having a low therapeutic index. Patients with epilepsy.
Side Effects
Side Effects
By frequency of occurrence, side effects are classified as follows:
Very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1000 to <1/100), rare (≥1/10000 to <1/1000), very rare, including isolated reports (<1/10000), frequency not established.
Immune system disorders
Hypersensitivity, urticaria. Frequency not set.
Nervous system disorders
Dizziness, headache. Frequency not set.
Gastrointestinal disorders
Rarely: nausea, abdominal pain, diarrhea, dyspepsia.
Skin and subcutaneous tissue disorders
Allergic reactions such as eczema, itching, rash. Frequency not set.
Vascular disorders
Bleeding from certain organs. Frequency not set.
If you experience the side effects listed in the instructions, or they get worse, or you notice any other side effects not listed in the instructions, tell your doctor.
Interaction
Interaction
Caution should be exercised when taking the drug in patients constantly taking acetylsalicylic acid and anticoagulants (direct and indirect). Isolated cases of bleeding are possible in patients simultaneously taking drugs that reduce blood clotting; the cause-and-effect relationship of these bleedings with the use of Ginkgo biloba preparations has not been confirmed.
The simultaneous use of Ginkgo biloba preparations with efavirenz is not recommended, since its concentration in the blood plasma may decrease due to the induction of cytochrome CYP3A4 under the influence of Ginkgo biloba extract.
Overdose
Overdose
Cases of drug overdose have not been reported to date. Memoplant is well tolerated.
Storage conditions
Storage conditions
Store at a temperature not exceeding 30 °C.
Keep out of the reach of children.
Shelf life
Shelf life
5 years.
Do not use after expiration date!
Manufacturer
Manufacturer
Dr. Wilmar Schwabe GmbH & Co. KG, Germany
Additional information
| Shelf life | 5 years. Do not use after the expiration date! |
|---|---|
| Conditions of storage | Store at the temperature not more than 30 ° C. Keep out of reach of children. |
| Manufacturer | Dr. Willmar Schwabe GmbH & Co. KG, Germany |
| Medication form | pills |
| Brand | Dr. Willmar Schwabe GmbH & Co. KG |
Other forms…
Related products
Buy Memoplant, 120 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.