No products in the cart.
Gingkoom, 40 mg capsules 90 pcs
€21.58 €17.99
Description
Pharmacotherapeutic group Anhydroprotective agent of plant origin
ATX code: N06DX02
Pharmacological properties
Enhances the body’s resistance to hypoxia, especially brain tissue. Inhibits the development of traumatic or toxic cerebral edema, improves cerebral and peripheral blood circulation, improves blood rheology (prevents thrombosis).
It has a dose-dependent regulatory effect on the vascular wall, dilates small arteries and increases the tone of veins. Prevents the formation of free radicals and lipid peroxidation in cell membranes. Normalizes the release, re-uptake and catabolism of neurotransmitters (norepinephrine, dopamine, acetylcholine) and their ability to combine with receptors. It improves metabolism in organs and tissues, promotes the accumulation of macroergens in cells, increased utilization of oxygen and glucose and normalization of mediator processes in the central nervous system.
Pharmacokinetics
absorption
After oral administration, the bioavailability of the terpenlactones (ginkgolide A, ginkgolide B, and bilobalide) is 80% for ginkgolide A, 88% for ginkgolide B, and 79% for bilobalide.
Distribution
The peak plasma concentration was 16-22 ng/mL for ginkgolide A, 8-10 ng/mL for ginkgolide B, and 27-54 ng/mL for bilobalide after oral tablets. Binding to plasma proteins is 43% for ginkgolide A, 47% for ginkgolide B and 67% for bilobalide.
Evolution
The elimination half-life is 3.9 hours for ginkgolide A, 4-6 hours for ginkgolide B and 2-3 hours for bilobalide.
Indications
Indications
Symptomatic treatment of cognitive impairment in adults (memory deterioration, decreased concentration and intellectual abilities).
As part of complex therapy for dizziness of vestibular origin as an auxiliary treatment in addition to vestibular rehabilitation.
Symptomatic treatment of tinnitus (ringing or noise in the ears).
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group Angioprotective agent of plant origin
ATX code: N06DX02
Pharmacological properties
Pharmacodynamics
Increases the body’s resistance to hypoxia, especially brain tissue. Inhibits the development of traumatic or toxic cerebral edema, improves cerebral and peripheral circulation, improves blood rheology (prevents thrombus formation).
It has a dose-dependent regulatory effect on the vascular wall, dilates small arteries, and increases the tone of the veins. Prevents the formation of free radicals and lipid peroxidation of cell membranes. Normalizes the release, re-uptake and catabolism of neurotransmitters (norepinephrine, dopamine, acetylcholine) and their ability to bind to receptors. Improves metabolism in organs and tissues, promotes the accumulation of macroergs in cells, increases the utilization of oxygen and glucose, and normalizes mediator processes in the central nervous system.
Pharmacokinetics
Suction
After oral administration, the bioavailability of the terpene lactones (ginkgolide A, ginkgolide B and bilobalide) is 80% for ginkgolide A, 88% for ginkgolide B and 79% for bilobalide.
Distribution
Peak plasma concentrations were 16–22 ng/mL for ginkgolide A, 8–10 ng/mL for ginkgolide B, and 27–54 ng/mL for bilobalide after oral tablet administration. Plasma protein binding is 43% for ginkgolide A, 47% for ginkgolide B and 67% for bilobalide.
Removal
The half-life is 3.9 hours for ginkgolide A, 4-6 hours for ginkgolide B and 2-3 hours for bilobalide.
Special instructions
Special instructions
Do not exceed the recommended doses of the drug.
If a hypersensitivity reaction develops, the use of the drug should be discontinued. Before surgery, you must inform your doctor about the use of the drug.
If you experience frequent feelings of dizziness and tinnitus, you should consult a doctor. In case of sudden deterioration or loss of hearing, you should immediately consult a doctor.
Patients with bleeding disorders (hemorrhagic diathesis) and patients receiving anticoagulant therapy should consult a physician before starting drug therapy.
It is not recommended to take the drug together with ethanol.
When using the drug in patients suffering from epilepsy, epileptic seizures may occur.
Impact on the ability to drive vehicles and machinery
During the period of taking the drug, care should be taken when performing potentially hazardous activities that require increased concentration and speed of psychomotor reactions (including driving vehicles, working with moving mechanisms), and if adverse reactions develop, including dizziness, it is necessary to refrain from driving.
Active ingredient
Active ingredient
Ginkgo biloba leaf extract
Composition
Composition
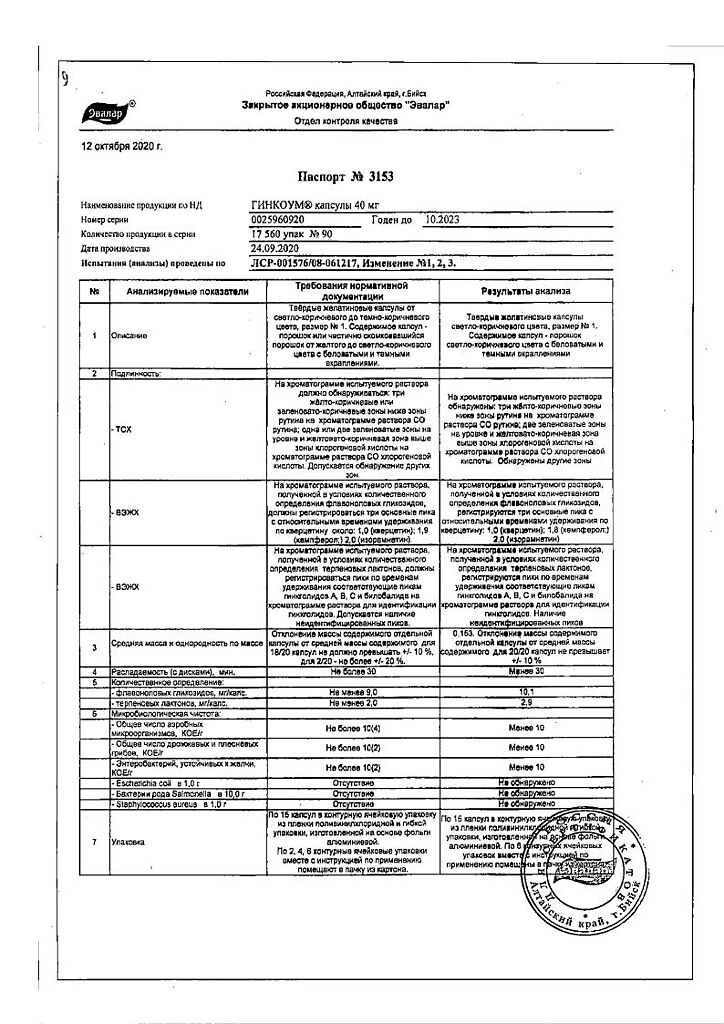
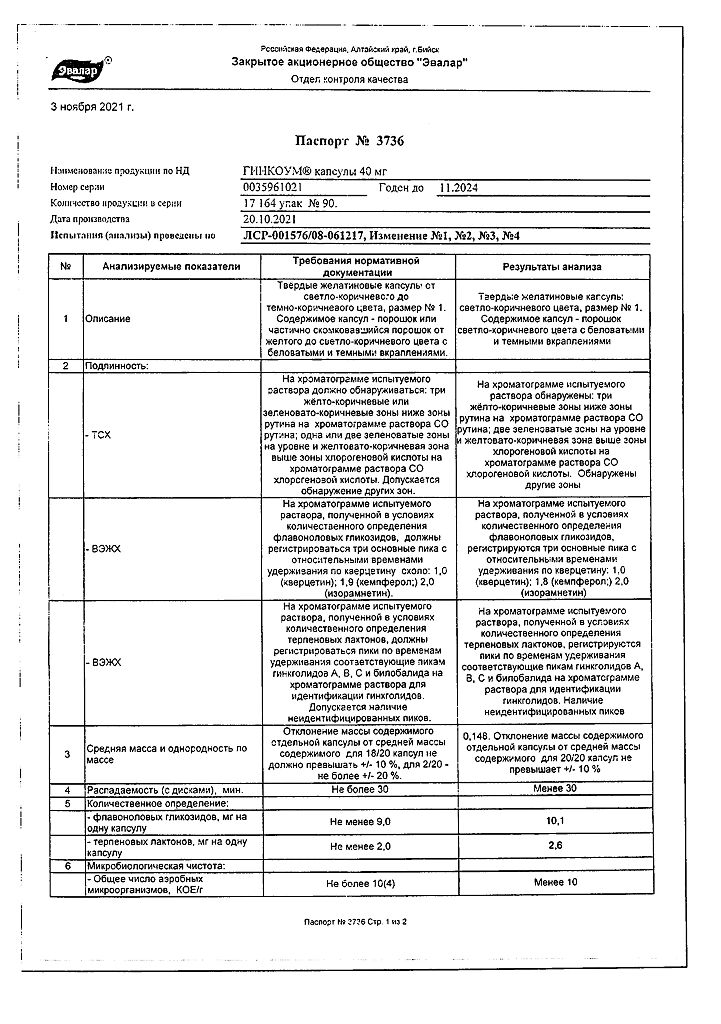
active ingredient: ginkgo biloba dry extract, standardized with a content of flavonol glycosides 22.0-27.0% and terpene lactones 5.0-12.0% -0.040 g.
excipients: microcrystalline cellulose – 0.109 g, calcium stearate – 0.001 g, hard gelatin capsules (capsule body components: black iron oxide dye E 172, red iron oxide dye E 172, titanium dioxide E 171, yellow iron oxide dye E 172, gelatin;
components of the capsule cover: iron dye black oxide E 172, iron oxide red dye E 172, titanium dioxide E 171, iron oxide yellow dye E 172, gelatin).
Pregnancy
Pregnancy
Due to the lack of sufficient clinical data, the use of the drug is contraindicated during pregnancy and breastfeeding.
Contraindications
Contraindications
Hypersensitivity to the components of the drug, decreased blood clotting, erosive gastritis in the acute stage, peptic ulcer of the stomach and duodenum in the acute stage, acute cerebrovascular accidents, acute myocardial infarction, pregnancy and breastfeeding, children under 18 years of age (due to the lack of sufficient clinical data).
With caution
Use in patients with epilepsy; with arterial hypotension.
Side Effects
Side Effects
Classification of the incidence of side effects according to the recommendations of the World Health Organization (WHO):
very often
≥ 1/10
often
from ≥ 1/100 to < 1/10
infrequently
from ≥ 1/1000 to < 1/100
rarely
from ≥ 1/10000 to < 1/1000
very rarely
from < 1/10000
frequency unknown
cannot be estimated based on the available data.
Within each group, adverse effects are presented in decreasing order of severity.
From the digestive system:
very rarely: nausea, vomiting, diarrhea.
From the nervous system:
very rarely: headache, dizziness, insomnia.
Allergic reactions:
very rarely: hyperemia, swelling, skin itching.
From the hemostasis system:
very rarely: decreased blood clotting. There are reports of bleeding occurring during long-term use of ginkgo biloba preparations in patients who were simultaneously taking anticoagulants.
From the senses:
frequency unknown: hearing impairment.
If any adverse events occur, you should stop taking the drug and consult your doctor.
Interaction
Interaction
The use of the drug is not recommended for patients constantly taking acetylsalicylic acid, anticoagulants (direct and indirect action), as well as thiazide diuretics, tricyclic antidepressants, anticonvulsants, gentamicin. Isolated cases of bleeding are possible in patients simultaneously taking medications that reduce blood clotting; the cause-and-effect relationship of these bleedings with taking ginkgo biloba preparations has not been confirmed.
When used with antihypertensive drugs, the antihypertensive effect may be potentiated.
The simultaneous use of ginkgo biloba preparations with efavirenz is not recommended, since a decrease in its concentration in the blood plasma is possible due to the induction of the cytochrome CYP3A4 isoenzyme under the influence of ginkgo biloba extract.
An interaction study with talinolol suggests that ginkgo biloba extract may inhibit intestinal P-glycoproteins. This may increase the intestinal exposure of P-gp-sensitive drugs such as dabigatran and should be used with caution during concomitant use.
Overdose
Overdose
Cases of drug overdose have not been reported to date. In case of overdose, treatment is symptomatic.
Action
Action
100% natural medicine Ginkoum:
• Improves cerebral circulation, restoring the supply of oxygen and glucose to the brain;
• Reduces blood viscosity and improves its fluidity, thereby preventing the formation of blood clots – the main cause of strokes;
• Improves metabolic processes in nerve cells, helping to improve memory, attention, mental activity and speed of thinking at any age2;
• Protects the walls of blood vessels from damage, normalizes the tone of arteries and veins;
• Protects the brain from free radicals.
Storage conditions
Storage conditions
At a temperature not exceeding 25 °C, in the original packaging.
Keep out of the reach of children.
Shelf life
Shelf life
3 years.
Do not use after expiration date.
Manufacturer
Manufacturer
Evalar CJSC, Russia
Additional information
| Shelf life | 3 years. Do not use after the expiration date. |
|---|---|
| Conditions of storage | At a temperature not exceeding 25 ° C, in the original package. Keep out of reach of children. |
| Manufacturer | Evalar, Russia |
| Medication form | capsules |
| Brand | Evalar |
Other forms…
Related products
Buy Gingkoom, 40 mg capsules 90 pcs with delivery to USA, UK, Europe and over 120 other countries.