No products in the cart.
Xarelto, 2,5mg 28 pcs.
€1.00
Out of stock
(E-mail when Stock is available)
Description
Rivaroxaban is a highly selective direct factor Xa inhibitor with high bioavailability when taken orally.
The activation of factor X to form factor Xa through the internal and external clotting pathways plays a central role in the coagulation cascade. Factor Xa is a component of the prothrombinase complex that forms, the action of which leads to the conversion of prothrombin to thrombin.
These reactions result in the formation of fibrin thrombus and platelet activation by thrombin. One molecule of factor Xa catalyzes the formation of over 1000 molecules of thrombin, which has become known as the “thrombin burst”. The reaction rate of prothrombinase bound factor Xa increases 300,000 times that of free factor Xa, providing a dramatic jump in thrombin levels. Selective factor Xa inhibitors can stop the “thrombin burst”. Thus, rivaroxaban affects the results of some specific or general laboratory tests used to evaluate clotting systems.
Pharmacodynamic effects
Dose-dependent inhibition of factor Xa has been observed in humans. Rivaroxaban has a dose-dependent effect on changes in prothrombin time, which correlates closely with the plasma concentration of rivaroxaban (correlation coefficient 0.98) when the Neoplastin® kit is used for analysis.
When other reagents are used the results will be different. Prothrombin time should be measured in seconds, because MHO is only calibrated and certified for coumarin derivatives and cannot be used for other anticoagulants. In patients undergoing major orthopedic surgery, the 5/95 percentiles for prothrombin time (Neoplastin®) 2 to 4 h after taking the tablet (i.e., at maximum effect) range from 13 to 25 seconds.
Rivaroxaban also dose-dependently increases the ABTV and HepTest® result; however, these parameters are not recommended for assessing the pharmacodynamic effects of rivaroxaban.
Monitoring of clotting parameters is not required during treatment with Xarelto®. However, if clinically warranted, rivaroxaban concentrations may be measured using a calibrated quantitative anti-Factor Xa test.
In healthy men and women over 50 years of age, no prolongation of the QT interval on the ECG has been observed with rivaroxaban.
Indications
Indications
Active ingredient
Active ingredient
Composition
Composition
How to take, the dosage
How to take, the dosage
Orally.
Xarelto® 2.5 mg should be taken 1 tablet 2 times daily with or without meals.
Prevention of cardiovascular death, myocardial infarction, and stent thrombosis in patients after ACS
After ACS, the recommended regimen for prevention of vascular events is 1 tablet of Xarelto® 2.5 mg 2 times daily. Patients should also take a daily dose of acetylsalicylic acid 75-100 mg or a daily dose of acetylsalicylic acid 75-100 mg in combination with a daily dose of clopidogrel 75 mg or a standard daily dose of ticlopidine.
The ongoing treatment should be regularly evaluated in terms of the balance between the risk of coronary events and the risk of bleeding. The duration of treatment is 12 months. Treatment may be extended up to 24 months for individual patients, as there are limited data on treatment of this duration.
The treatment with Xarelto® 2.5 mg should be initiated as soon as possible after the patient is stabilized during the current ACS (including revascularization procedures). Treatment with Xarelto® should be initiated at least 24 h after hospitalization. Xarelto® 2.5 mg should be started when parenteral anticoagulant therapy is usually discontinued.
Prevention of stroke, myocardial infarction and death due to cardiovascular causes, as well as prevention of acute limb ischemia and overall mortality in patients with CHD or VTE
Prevention of stroke, myocardial infarction and death due to cardiovascular causes/p>
The recommended dosing regimen for the prevention of vascular events in patients with CHD or ZPA is 1 tablet of Xarelto® 2.5 mg 2 times/day in combination with a daily dose of acetylsalicylic acid 75-100 mg. Treatment with Xarelto® 2.5 mg should be long-term, provided that the benefits obtained outweigh the risks.
In patients with an acute thrombotic event or vascular intervention requiring dual antiplatelet therapy, the need for continued Xarelto® 2.5 mg 2 times daily should be evaluated depending on the type of thrombotic event or procedure and the antiplatelet therapy. The safety and efficacy of Xarelto® 2.5 mg when taken 2 times/day in combination with acetylsalicylic acid and clopidogrel or ticlopidine have been studied only in patients with recent ACS. The use of dual antiplatelet therapy in combination with Xarelto® 2.5 mg 2 times/day in patients with CHD or OSA has not been studied.
In patients who have been diagnosed with CHD or ZPA, treatment with Xarelto® 2. 5 mg 2 times/day in combination with acetylsalicylic acid 75-100 mg once daily may be initiated at any time.
If a dose is missed, the patient should continue Xarelto® 2.5 mg at the usual dose, which is the next scheduled appointment as recommended.
If the patient is unable to swallow the tablet whole, the Xarelto® tablet may be crushed or mixed with water or a liquid food such as apple puree immediately before intake. The crushed tablet of Xarelto® may be administered through a gastric tube. The position of the probe in the gastrointestinal tract should be additionally coordinated with the doctor before taking Xarelto®. The crushed tablet should be inserted through the gastric tube in a small amount of water, after which a small amount of water should be injected to wash the drug residues from the walls of the tube.
Additional information for special patient groups
Patients with impaired liver function
The drug Xarelto
sup>® is contraindicated in patients with liver disease with coagulopathy leading to a clinically significant risk of bleeding.
Patients with other liver diseases do not require dose adjustment.
Limited clinical data obtained in patients with moderate hepatic impairment (Child-Pugh class B) indicate a significant increase in pharmacological activity. No clinical data are available for patients with severe hepatic impairment (Child-Pugh class C).
Patients with impaired renal function
Dose adjustment is not required if Xarelto® is used in patients with mild (CK 50-80 ml/min) or moderate (CK 30-49 ml/min) impaired renal function.
Limited clinical data obtained in patients with severe renal dysfunction (KC 15-29 mL/min) indicate that plasma concentrations of rivaroxaban are significantly elevated in this patient population. Therefore, Xarelto® should be used with caution in these patients.
The use of Xarelto® is not recommended in patients with CK < 15 ml/min.
The switch from vitamin K antagonists (VKAs) to Xarelto®
The switch of patients from VKAs to Xarelto® INR values will be erroneously elevated after taking Xarelto®. The INR is not suitable for determining the anticoagulant activity of Xarelto® and therefore should not be used for this purpose.
Transition from Xarelto® to therapy with vitamin K antagonists (VKAs)
There is a possibility of inadequate anticoagulant activity with Xarelto®. There is a possibility of insufficient anticoagulant effect when switching from Xarelto® therapy to AVC therapy. Therefore, it is necessary to ensure continuous sufficient anticoagulant effect during such transition with alternative anticoagulants. It should be noted that when switching from Xarelto® to AVC therapy, Xarelto® may contribute to increased INR.
In patients transitioning from Xarelto® therapy to AVC therapy, the latter should be taken continuously until the INR is â¥2.0. For the first two days of the transition period, AVCs should be used in standard doses, subsequently adapting the dose of AVCs according to the INR value. Since patients receive both Xarelto® and AVC at the same time during this period, the INR should be assessed no earlier than 24 hours (after the first dose, but before the next dose of Xarelto®). Thus, after discontinuation of Xarelto®, INR may not be used until 24 hours after the last dose of Xarelto® as a reliable assessment of the therapeutic effect of AVC.
Transition from therapy with parenteral anticoagulants to therapy with Xarelto®
For patients receiving parenteral anticoagulants, the use of Xarelto® should be started 0-2 hours before the time of the next scheduled parenteral administration of the drug (e.g., low molecular weight heparin) or at the time of discontinuation of continuous parenteral administration of the drug (e.g., intravenous infusion of unfractionated heparin).
Transition from therapy with Xarelto® to therapy with parenteral anticoagulants
The drug Xarelto
should be discontinued.sup>® and administer the first dose of parenteral anticoagulant at the time the next dose of Xarelto® should have been taken.
Children and adolescents (birth to 18 years)
The safety and effectiveness of use in children and adolescents under 18 years has not been established.
Elderly patients
There is no need to adjust the dose according to age.
Gender
Dose adjustment depending on gender is not required.
Body weight
Dose adjustment depending on body weight is not required.
Ethnicity
Dose adjustment based on ethnicity is not required.
Interaction
Interaction
Pharmacokinetic interaction
Excretion of rivaroxaban is primarily by metabolism in the liver mediated by the cytochrome P450 system (CYP3A4, CYP2J2) and also by renal excretion of unchanged drug using the P-gp/Bcrp (P-glycoprotein/milk cancer resistance protein) carrier systems.
CYP inhibition
Rivaroxaban does not inhibit CYP3A4 or any other major CYP isoenzymes.
Induction of CYP
Rivaroxaban does not induce CYP3A4 or any other major CYP isoenzymes.
The effects on rivaroxaban
The concomitant use of rivaroxaban and potent CYP3A4 and P-glycoprotein isoenzyme inhibitors may decrease the renal and hepatic clearance of rivaroxaban and thus significantly increase its systemic effects.
The co-administration of rivaroxaban and the azole antifungal agent ketoconazole (400 mg once daily), a potent CYP3A4 and P-glycoprotein inhibitor, resulted in a 2.6-fold and increased the mean Cmax of rivaroxaban by 1.7-fold, which was accompanied by a significant increase in the pharmacodynamic action of the drug.
The co-administration of rivaroxaban and the HIV protease inhibitor ritonavir (600 mg 2 times/day), a potent CYP3A4 and P-glycoprotein inhibitor, resulted in a 2.5-fold and increased the mean Cmax of rivaroxaban by 1.6-fold, which was accompanied by a significant increase in the pharmacodynamic action of the drug. Therefore, rivaroxaban is not recommended for use in patients receiving systemic treatment with antifungal drugs of azole group or HIV protease inhibitors.
Clarithromycin (500 mg 2 times daily), a potent CYP3A4 isoenzyme inhibitor and moderate P-glycoprotein inhibitor, caused 1.5-fold increase in AUC values and 1.4-fold increase in Cmax rivaroxaban. This increase is of the order of the normal variability of AUC and Cmax and is considered clinically insignificant.
Eritromycin (500 mg 3 times/day), a moderate inhibitor of the CYP3A4 isoenzyme and P-glycoprotein, caused a 1.3-fold increase in the AUC and Cmax values of rivaroxaban. This increase is of the order of the normal variability of AUC and Cmax and is considered clinically insignificant.
In patients with mild renal dysfunction (CK 50-80 mL/min), erythromycin (500 mg 3 times/day) caused a 1.8-fold increase in rivaroxaban AUC and 1.6-fold increase in Cmax values compared with patients with normal renal function who did not receive concomitant therapy. In patients with moderate renal function impairment (CKR 30-49 mL/min), erythromycin caused a 2.0-fold increase in rivaroxaban AUC and 1.6-fold increase in Cmax compared to patients with normal renal function who did not receive concomitant therapy.
Fluconazole (400 mg once daily), a moderate CYP3A4 isoenzyme inhibitor, caused a 1.4-fold increase in mean AUC of rivaroxaban and a 1.3-fold increase in mean Cmax. This increase is of the order of normal AUC variability and Cmax is considered clinically insignificant.
The concomitant use of rivaroxaban with dronedarone should be avoided due to limited clinical data on co-administration.
The co-administration of Xarelto® and rifampicin, which is a potent inducer of CYP3A4 and P-glycoprotein, resulted in a reduction of the mean AUC of rivaroxaban by approximately 50% and a concomitant reduction of its pharmacodynamic effects. Co-administration of rivaroxaban with other potent CYP3A4 inducers (e.g., phenytoin, carbamazepine, phenobarbital or preparations of St. John’s wort) may also result in decreased plasma concentrations of rivaroxaban. Decreased plasma concentrations of rivaroxaban have been found to be clinically insignificant. Powerful CYP3A4 inducers should be used with caution.
No pharmacokinetic interaction has been reported between warfarin and Xarelto®.
Pharmacodynamic interaction
After concomitant use of enoxaparin sodium (single dose of 40 mg) and Xarelto® (single dose 10 mg), there was a summated effect on anti-Factor Xa activity, with no additional summated effects on clotting tests (prothrombin time, ACTV). Enoxaparin did not alter the pharmacokinetics of rivaroxaban.
Due to the increased risk of bleeding, caution should be exercised when coadministering with any other anticoagulants.
No pharmacokinetic interaction has been found between Xarelto® (15 mg) and clopidogrel (a loading dose of 300 mg followed by a maintenance dose of 75 mg), but a significant increase in bleeding time was found in a subgroup of patients that did not correlate with the degree of platelet aggregation and P-selectin or GPIIb/IIIa-receptor content.
After co-administration of Xarelto® (15 mg) and naproxen (500 mg) no clinically significant increase in bleeding time was observed. However, a more pronounced pharmacodynamic response is possible in individuals.
Caution should be exercised when co-administering Xarelto® with NSAIDs (including acetylsalicylic acid) and platelet aggregation inhibitors, as use of these drugs generally increases the risk of bleeding.
The conversion of patients from warfarin (MHO 2 to 3) to Xarelto® (20 mg) or from Xarelto® (20 mg) to warfarin (MHO 2 to 3) increased prothrombin time/MNO (Neoplastin) to a greater extent than would be expected by simply summing the effects (individual MHO values as high as 12), whereas the effects on AChTV, suppression of factor Xa activity and endogenous thrombin potential were additive.
If the pharmacodynamic effects of Xarelto® should be investigated during the transition period, anti-Xa activity, PiCT and HepTest determination may be used as necessary tests that are not affected by warfarin. From day 4 after discontinuation of warfarin, all test results (including PV, ACTV, inhibition of factor Xa activity, and on EPT (endogenous thrombin potential)) reflect only the effects of Xarelto®.
If it is necessary to investigate the pharmacodynamic effects of warfarin during the transition period, the measurement of MHO at Cinterval may be used. rivaroxaban (24 h after the previous dose of rivaroxaban), because rivaroxaban has minimal effect on this index during this period.
The drug interaction of Xarelto® with phenyndione AVC has not been studied. It is recommended to avoid transferring patients from Xarelto® therapy to AVC therapy with phenyndione and vice versa whenever possible. There is limited experience transferring patients from AVC therapy with acenocoumarol to Xarelto®.
If it becomes necessary to transfer a patient from Xarelto® therapy to AVC therapy with phenyndione or acenocoumarol, special care should be taken while co-administering Xarelto® and AVC, daily monitoring of pharmacodynamic effects of the drugs (MHO, prothrombin time) should be performed immediately before the next dose of Xarelto®. If it is necessary to switch a patient from AVC therapy with phenindion or acenocoumarol to therapy with Xarelto®, special caution should be exercised and no pharmacodynamic monitoring of the drugs is required.
As with other anticoagulants, caution should be exercised when Xarelto® is coadministered with selective serotonin reuptake inhibitors (SSRIs) and selective serotonin and norepinephrine reuptake inhibitors (SNRIs), as use of these drugs increases the risk of bleeding. Results of clinical trials have demonstrated a numerical increase in major and minor clinically significant bleeding in all treatment groups when these drugs are used together.
Foods and dairy products
The drug Xarelto® can be taken regardless of meals.
No interactions have been identified
No pharmacokinetic interactions have been identified between rivaroxaban and midazolam (CYP3A4 substrate), digoxin (P-glycoprotein substrate) or atorvastatin (CYP3A4 and P-glycoprotein substrate).
The co-administration with the proton pump inhibitor omeprazole, histamine H2-receptor blocker ranitidine, aluminum hydroxide/magnesium hydroxide antacids, naproxen, clopidogrel or enoxaparin does not affect bioavailability and pharmacokinetics of rivaroxaban.
No clinically significant pharmacokinetic or pharmacodynamic interactions have been observed when Xarelto® and 500 mg acetylsalicylic acid are used together.
Impact on laboratory parameters
The drug Xarelto® affects parameters of blood clotting (prothrombin time, ACTV, Hep-Test) due to its mechanism of action.
Special Instructions
Special Instructions
The use of concomitant drugs
Rivaroxaban is not recommended in patients receiving concomitant systemic treatment with azole antifungals (e.g., ketoconazole) or HIV protease inhibitors (e.g., ritonavir). These drugs are strong inhibitors of CYP3A4 and P-glycoprotein. Thus, these drugs may increase plasma concentrations of rivaroxaban to clinically significant levels (2.6-fold on average), which may lead to an increased risk of bleeding. However, the azole antifungal drug fluconazole, a moderate CYP3A4 inhibitor, has a less pronounced effect on the exposure of rivaroxaban and can be used simultaneously with it.
Rivaroxaban should be used with caution in patients with moderate renal impairment (CKR 30-49 mL/min) receiving concomitant medications that may increase plasma concentrations of rivaroxaban.
In patients with severe renal impairment (CK< 30 ml/min), plasma concentrations of rivaroxaban may be significantly elevated (1.6-fold on average), which may lead to an increased risk of bleeding. Therefore, due to the presence of the indicated underlying disease, these patients have an increased risk of developing both bleeding and thrombosis. Due to limited clinical data, rivaroxaban should be used with caution in patients with CK of 15-29 ml/min.
There are no clinical data on the use of rivaroxaban in patients with severe renal impairment (CK< 15 ml/min). Therefore, the use of Xarelto® is not recommended in these patients.
Patients with severe renal impairment (CK<15-29 ml/min), increased risk of bleeding, as well as patients receiving concomitant systemic treatment with azole antifungal agents or HIV protease inhibitors should be monitored closely for signs of bleeding after initiation of treatment. Follow-up can be accomplished by regular physical examination of patients, close monitoring of postoperative wound drainage, and periodic hemoglobin testing.
Patients with a history of stroke and/or TIA
The administration of Xarelto® at a dose of 2.5 mg 2 times/day is contraindicated in patients with ACS who have a history of stroke or TIA. Only a few ACS patients with a history of stroke or TIA have been studied, but the limited data demonstrate no clinical benefit of rivaroxaban treatment in such patients.
Patients with a history of hemorrhagic or lacunar stroke
Patients with CHD or TIA with a history of hemorrhagic or lacunar stroke have not been studied. Treatment with Xarelto® 2.5 mg 2 times/day in combination with acetylsalicylic acid is contraindicated in these patients.
Patients with ischemic non-lacunar stroke
Patients with CHD or OSA who have had an ischemic non-lacunar stroke in the previous month have not been studied. Treatment with Xarelto® 2.5 mg 2 times daily in combination with acetylsalicylic acid is contraindicated in such patients during the first month after stroke.
The risk of bleeding
The drug Xarelto®, like other antithrombotic agents, should be used with caution in patients who have an increased risk of bleeding, such as:
Features
Features
Introduction
After oral administration rivaroxaban is quickly and almost completely absorbed. Cmax is reached 2-4 h after taking the tablet. Bioavailability of rivaroxaban when taking 2.5 mg tablets is high (80-100%) regardless of food intake. Food intake has no effect on AUC and Cmax when taking the drug at a dose of 10 mg. Xarelto® Tablets in 2.5 mg dosage can be taken with food as well as on an empty stomach.
The pharmacokinetics of rivaroxaban are characterized by moderate interindividual variability, with a Cv% coefficient of variability of 30% to 40%.
The absorption of rivaroxaban depends on the site of release in the GI tract. A reduction in AUC and Cmax of 29% and 56%, respectively, compared to whole tablet administration was observed when rivaroxaban pellet was administered in the proximal small intestine. Exposure to the drug is also reduced when administered into the distal small intestine or the ascending colon. Administration of rivaroxaban in the GI tract distal to the stomach should be avoided because it may result in reduced absorption and, therefore, exposure of the drug.
The bioavailability (AUC and Cmax) of rivaroxaban 20 mg when taken as a whole tablet is comparable to the bioavailability of the drug taken orally as a crushed tablet (mixed with apple puree or suspended in water) and to the bioavailability of the drug when administered via gastric tube followed by a liquid meal. Given the predictable dose-dependent pharmacokinetic profile of rivaroxaban, the results of this bioavailability study are also applicable to lower doses.
Distribution
Rivaroxaban has a high degree of binding to plasma proteins of approximately 92-95%, with the bulk of rivaroxaban binding to serum albumin. The drug has an average Vd of approximately 50L.
Metabolism
In oral administration, approximately 2/3 of the received dose of rivaroxaban is metabolized and excreted by the kidneys and through the intestine in equal proportions. The remaining 1/3 of the dose received is excreted via direct renal excretion unchanged, primarily through active renal secretion.
Rivaroxaban is metabolized by CYP3A4, CYP2J2 isoenzymes as well as by mechanisms independent of the cytochrome system. The main sites of biotransformation are oxidation of the morpholine group and hydrolysis of the amide bonds.
In vitro data show that rivaroxaban is a substrate for P-gp (P-glycoprotein) and Bcrp (breast cancer resistance protein) carrier proteins.
Unchanged rivaroxaban is the only active compound in plasma; no major or active circulating metabolites were detected in plasma.
Elimination
Rivaroxaban, with a systemic clearance of approximately 10 L/h, may be classified as a low clearance drug. In plasma excretion of rivaroxaban, the final T1/2 is 5 to 9 h in young patients and 11 to 13 h in elderly patients.
Pharmacokinetics in special clinical cases
Period/older age (older than 65 years). Elderly patients have higher plasma concentrations of rivaroxaban than younger patients; the average AUC is approximately 1.5 times greater than in younger patients, primarily due to seemingly decreased total and renal clearance.
There were no clinically significant differences in pharmacokinetics between men and women.
Body weight. Too little or too much body weight (less than 50 kg and more than 120 kg) only slightly affects the plasma concentration of rivaroxaban (less than 25% difference).
Childhood and adolescence (from birth to 18 years). No data are available for this age group.
Interethnic differences. No clinically significant differences in pharmacokinetics and pharmacodynamics were observed in patients of Caucasian, African American, Hispanic, Japanese, or Chinese ethnicity.
Patients with impaired hepatic function. The effect of hepatic impairment on the pharmacokinetics of rivaroxaban has been studied in patients classified according to the Child-Pugh classification (according to standard procedures in clinical trials). The Child-Pugh classification provides an assessment of the prognosis of chronic liver disease, mainly cirrhosis. In patients scheduled for anticoagulant therapy, the most important consequence of impaired liver function is reduced synthesis of clotting factors in the liver. Because this figure corresponds to only one of the five clinical/biochemical criteria that make up the Child-Pugh classification, the risk of bleeding does not correlate clearly with this classification. Treatment of such patients with anticoagulants should be decided regardless of the Child-Pugh classification.
Rivaroxaban Xarelto® is contraindicated in patients with liver disease presenting with coagulopathy causing clinically significant bleeding risk.
In patients with cirrhosis with mild hepatic impairment (Child-Pugh Class A) the pharmacokinetics of rivaroxaban were only slightly different from those in the control group of healthy subjects (on average there was 1.2-fold AUC increase of rivaroxaban). There were no significant differences in pharmacodynamic properties between the groups.
In patients with cirrhosis and moderate hepatic impairment (Child-Pugh class B), the mean AUC of rivaroxaban was significantly increased (2.3-fold) compared to healthy volunteers due to significantly reduced drug clearance, indicating severe liver disease. Suppression of factor Xa activity was more pronounced (2.6-fold) than in healthy volunteers. Prothrombin time was also 2.1 times that of healthy volunteers. Prothrombin time measurement assesses the external coagulation pathway, which includes clotting factors VII, X, V, II and I, which are synthesized in the liver. Patients with moderate hepatic impairment are more sensitive to rivaroxaban, which is a consequence of a closer relationship between pharmacodynamic effects and pharmacokinetic parameters, especially between concentration and prothrombin time.
There are no data for patients with Child-Pugh class C hepatic impairment.
Patients with impaired renal function. In patients with impaired renal function, there was an increase in AUC inversely proportional to the degree of decrease in renal function as assessed by CK.
In patients with mild renal impairment (CKR 50-80 ml/min), moderate renal impairment (CKR 30-49 ml/min), and severe renal impairment (CKR 15-29 ml/min), a 1.4-, 1.5-, and 1.6-fold increase in plasma concentrations of rivaroxaban (AUC) compared to healthy volunteers was observed, respectively.
The corresponding increase in pharmacodynamic effects was more pronounced.
In patients with mild, moderate and severe renal impairment total suppression of factor Xa activity was increased by 1.5, 1.9 and 2 times in comparison with healthy volunteers; prothrombin time due to factor Xa activity was also increased by 1.3, 2.2 and 2.4 times respectively.
The data on the use of Xarelto® in patients with a CK of 29-15 ml/min are limited, and therefore caution should be exercised when using the drug in this patient population. There are no data on the use of Xarelto® in patients with IQ < 15 ml/min, therefore it is not recommended to use the drug in this category of patients.
Due to the underlying disease, patients with severe renal dysfunction are at high risk of bleeding and thrombosis.
Contraindications
Contraindications
Side effects
Side effects
The safety of Xarelto® was evaluated in twelve phase III studies including 53,103 patients taking Xarelto®.
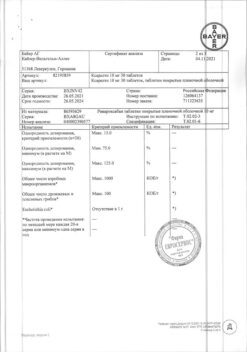
Table 1. Number of patients enrolled in the study who took at least 1 dose of rivaroxaban, total daily dose, and maximum duration of treatment in phase III clinical trials using Xarelto®
*Patients who have received at least one dose of rivaroxaban.
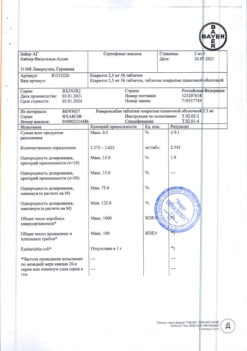
Table 2. Frequency of bleeding and anemia in patients treated with Xarelto® in phase III clinical trials
* A prespecified sampling approach was used to collect data on adverse events
Given the mechanism of action, the use of Xarelto® may be associated with an increased risk of occult or overt bleeding from any tissue or organ, which may lead to post-hemorrhagic anemia. The risk of bleeding may increase in patients with severe uncontrolled arterial hypertension and/or when co-administered with drugs that affect hemostasis.
The signs, symptoms and severity (including possible lethal outcome) will vary depending on the source and degree or severity of bleeding and/or anemia.
Hemorrhagic complications may manifest as weakness, pallor, dizziness, headache or unexplained edema, shortness of breath or shock that cannot be explained by other causes. In some cases, symptoms of myocardial ischemia, such as chest pain or angina, may occur as a consequence of anemia.
Xarelto® has also reported known complications secondary to severe bleeding, such as increased subfacial pressure syndrome (compartment syndrome) and renal failure due to hypoperfusion. Thus, the possibility of bleeding should be considered in the evaluation of any patient receiving anticoagulants.
The incidence of NCDs (adverse drug reactions) with Xarelto® is shown in the table below. Within each frequency group, adverse events are presented in decreasing order of severity.The frequency is defined as: very common (â¥1/10), common (â¥1/100 to < 1/10), infrequent (â¥1/1,000 to < 1/100), rare (â¥1/10,000 to < 1/1,000).
Table 3. All treatment-emergent adverse drug reactions reported in patients in phase III clinical trials (cumulative data from RECORD 1-4, ROCKET AF, J-ROCKET, MAGELLAN, ATLAS and EINSTEIN (DVT/PE/Extension/CHOICE) and COMPASS*)
/p> Often Infrequently . Rarely Blood and lymphatic system side Anemia (including
relevant
laboratory parameters) Thrombocytosis
(including increased
platelet count)A
Immune system side
Allergic reaction,
allergic dermatitis Respiratory system, thoracic and mediastinal organs nasal bleeding,
hemoptysis
Gastrointestinal side bleeding gums, nausea, constipationA, diarrhea,
vomitingA Dry mouth
. /td> Impaired liver function Jaundice Skeletal muscle and connective tissue side Pain in extremitiesA Hemarthrosis Muscle hemorrhage Local edemaA Trauma, intoxication, and complications after manipulation Wound secretion
from woundA Vascular
pseudoaneurysmC Laboratory and instrumental findings Increased activity
hepatic transaminases Increased concentration of
Overdose
Overdose
Rare cases of overdose have been reported when taking rivaroxaban up to 600 mg without the development of bleeding or other adverse reactions. Due to limited absorption, a concentration plateau is expected without further increase in the average plasma concentration of rivaroxaban when used at supratherapeutic doses (50 mg or higher).
Treatment: The specific antidote for rivaroxaban is unknown. In case of Xarelto® overdose, activated charcoal may be used to reduce absorption of rivaroxaban. Due to the significant binding of rivaroxaban to plasma proteins, rivaroxaban is not expected to be excreted by hemodialysis.
Treatment of bleeding
If a bleeding complication occurs, the next administration of the drug should be delayed or treatment with the drug should be stopped. T1/2 rivaroxaban is approximately 5-13 h. Treatment should be selected individually, depending on the severity and location of bleeding.
If necessary, appropriate symptomatic treatment such as mechanical compression (e.g., in severe nasal bleeding), surgical hemostasis with assessment of its effectiveness (bleeding control), infusion therapy and hemodynamic support, use of blood products (red blood cell mass or fresh frozen plasma, depending on whether anemia or coagulopathy has occurred) or platelets may be given.
If the above measures do not eliminate bleeding, specific reverse-acting procoagulant drugs such as prothrombin complex concentrate, activated prothrombin complex concentrate or recombinant factor VIIa may be used. However, there is currently limited experience with these drugs in patients receiving Xarelto®.
Protamine sulfate and vitamin K are not expected to affect the anticoagulant activity of rivaroxaban.
There is limited experience with tranexamic acid and no experience with aminocaproic acid and aprotinin in patients receiving Xarelto®. There is no scientific evidence or experience with the systemic hemostatic drug desmopressin in patients receiving Xarelto®.
Additional information
| Shelf life | 3 years. |
|---|---|
| Conditions of storage | The drug should be kept out of reach of children at a temperature not exceeding 30 ° C. |
| Manufacturer | Bayer AG, Germany |
| Medication form | pills |
| Brand | Bayer AG |
Other forms…
Related products
Buy Xarelto, 2,5mg 28 pcs. with delivery to USA, UK, Europe and over 120 other countries.