No products in the cart.
Xarelto, 15 mg 100 pcs
€284.26 €236.88
Out of stock
(E-mail when Stock is available)
Description
Pharmgroup:
Other antithrombotic agents – direct acting anticoagulant.
Pharm Action:
Rivaroxaban is a highly selective direct factor Xa inhibitor with high bioavailability when taken orally.
Activation of factor X to form factor Xa through the internal and external clotting pathways plays a central role in the coagulation cascade.
Pharmacodynamic effects.
Dose-dependent inhibition of factor Xa has been observed in humans. Rivaroxaban has a dose-dependent effect on prothrombin time and correlates closely with plasma concentrations (r=0.98) when using the Neoplastin® kit for analysis. Results will differ if other reagents are used. Prothrombin time should be measured in seconds because the INR (international normalized ratio) is calibrated and certified only for coumarin derivatives and cannot be used for other anticoagulants. In patients undergoing major orthopedic surgery, the 5/95 percentile for prothrombin time (Neoplastin®) 2 to 4 hours after taking the tablet (i.e., at peak effect) ranges from 13 to 25 seconds.
Rivaroxaban also dose-dependently increases the activated partial thromboplastin time (APTB) and HepTest® score; however, these parameters are not recommended for evaluating the pharmacodynamic effects of rivaroxaban.
Rivaroxaban also affects anti-Xa factor activity, but there are no standards for calibration.
Monitoring of clotting parameters is not required during treatment with rivaroxaban.
No QT interval prolongation has been observed in healthy men and women over 50 years of age under the influence of rivaroxaban.
Pharmacokinetics:
Absorption and bioavailability
The absolute bioavailability of rivaroxaban after a 10 mg dose is high (80-100%).
Rivaroxaban is rapidly absorbed; maximum concentration (Smax ) is reached 2-4 hours after taking the tablet.
No change in AUC (area under the concentration-time curve) and Smax (maximum concentration) was observed when rivaroxaban 10 mg was taken with food. Rivaroxaban in a dose of 10 mg may be administered with meals or independently of meals.
The pharmacokinetics of rivaroxaban are characterized by moderate individual variability; individual variability (coefficient of variation) ranges from 30% to 40%, except for the day of surgery and the following day, when variability in exposure is high (70%).
Distribution
In humans, most of rivaroxaban (92-95%) binds to plasma proteins, with serum albumin being the major binding component. The volume of distribution is moderate, Vss is approximately 50 liters.
Metabolism and excretion
When administered orally, approximately 2/3 of the prescribed dose of rivaroxaban is metabolized and subsequently excreted in equal parts with the urine and feces. The remaining one third of the dose is excreted by direct renal excretion unchanged, mainly due to active renal secretion.
Rivaroxaban is metabolized by CYP3A4, CYP2J2 isoenzymes as well as by mechanisms independent of the cytochrome system. The main sites of biotransformation are oxidation of the morpholine group and hydrolysis of the amide bonds.
According to in vitro data, rivaroxaban is a substrate for P-gp (P-glycoprotein) and Bcrp (breast cancer resistance protein) carrier proteins.
Unchanged rivaroxaban is the only active compound in human plasma; no significant or active circulating metabolites have been detected in plasma. Rivaroxaban, which has a systemic clearance of approximately 10 L/h, may
be classified as a low clearance drug. In plasma excretion of rivaroxaban, the final half-life is 5 to 9 hours in young patients and 11 to 13 hours in elderly patients.
Gender/Elderly (>65 years)
Elderly patients have higher plasma concentrations of rivaroxaban than younger patients, with average AUC values approximately 1.5 times greater than those in younger patients, primarily due to seemingly lower total and renal clearance.
No clinically significant differences in pharmacokinetics were found in men and women.
Body weight
Too little or too much body weight (less than 50 kg and more than 120 kg) only slightly affects the plasma concentration of rivaroxaban (the difference is less than 25%).
Children
Data are not available for this age group.
Interethnic differences
No clinically significant differences in pharmacokinetics and pharmacodynamics were observed in patients of Caucasian, African-American, Hispanic, Japanese, or Chinese ethnicity.
Hepatic impairment
The effect of hepatic impairment on the pharmacokinetics of rivaroxaban has been studied in patients graded according to the Child-Pugh classification (according to standard procedures in clinical trials). The Child-Pugh classification provides an assessment of the prognosis of chronic liver disease, mainly cirrhosis. In patients scheduled for anticoagulant therapy, a particularly critical point of impaired liver function is the decreased synthesis of clotting factors in the liver. Because this index corresponds to only one of the five clinical/biochemical criteria that make up the Child-Pugh classification, the risk of bleeding does not correlate clearly with this classification. Treatment of such patients with anticoagulants should be decided independently of the Child-Pugh classification.
Rivaroxaban is contraindicated in patients with liver disease presenting with coagulopathy causing clinically significant bleeding risk.
In patients with cirrhosis with mild hepatic impairment (Child-Pugh class A) the pharmacokinetics of rivaroxaban did not significantly differ (on the average there was 1.2-fold increase of AUC of rivaroxaban) from the corresponding parameters in control group of healthy subjects. There were no significant differences in pharmacodynamic properties between the groups.
In patients with cirrhosis and moderate hepatic impairment (Child-Pugh class B), the mean AUC of rivaroxaban was significantly elevated (2.3-fold) compared with healthy volunteers due to significantly reduced drug clearance indicative of severe liver disease. Suppression of factor Xa activity was more pronounced (2.6-fold) than in healthy volunteers. Prothrombin time was also 2.1 times that of healthy volunteers. Prothrombin time measurement assesses the external coagulation pathway, which includes clotting factors VII, X, V, II and I, which are synthesized in the liver. Patients with moderate hepatic insufficiency are more sensitive to rivaroxaban, which is a consequence of a closer relationship between pharmacodynamic effects and pharmacokinetic parameters, especially between concentration and prothrombin time.
There are no data available for patients with Child-Pugh class C hepatic impairment.
Renal impairment
In patients with renal impairment, increased plasma concentrations of rivaroxaban have been observed inversely correlated with decreased renal function as assessed by creatinine clearance.
In patients with mild (creatinine clearance 80-50 ml/min), moderate (creatinine clearance < 50-30 ml/min) or severe (creatinine clearance < 30-15 mL/min), a 1.4-, 1.5-, and 1.6-fold increase in plasma concentrations of rivaroxaban (AUC), respectively, was observed compared with healthy volunteers.
The corresponding increase in pharmacodynamic effects was more pronounced.
In patients with mild, moderate and severe renal impairment, total suppression of factor Xa activity was increased 1.5, 1.9 and 2-fold compared to healthy volunteers; prothrombin time due to factor Xa activity was also increased 1.3, 2.2 and 2.4-fold, respectively.
Data on the use of rivaroxaban in patients with a creatinine clearance of 30-15 ml/min are limited; therefore, caution should be exercised when using the drug in this patient population. There are no data on the use of rivaroxaban in patients with creatinine clearance < 15 ml/min, therefore it is not recommended to use the drug in this category of patients.
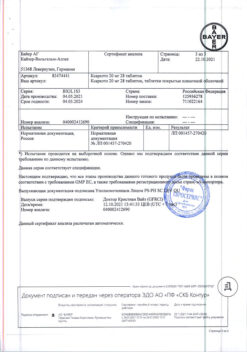
Indications
Indications
– Prevention of venous thromboembolism (VTE) in patients undergoing major orthopedic surgery on the lower extremities (for Xarelto 10 mg tablets).
Prevention of stroke and systemic thromboembolism in patients with non-valvular atrial fibrillation (for Xarelto 15 mg and 20 mg tablets).
Active ingredient
Active ingredient
Composition
Composition
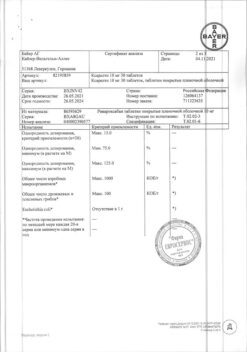
– rivaroxaban (micronized) 15 mg
Excipients:
microcrystalline cellulose – 37.5 mg,
croscarmellose sodium – 3 mg,
hypromellose 5cP – 3 mg,
lactose monohydrate – 25.4 mg,
magnesium stearate – 600 µg,
sodium lauryl sulfate – 500 µg.
Capsule composition:
Red iron oxide dye – 150 µg,
Hypromellose 15cP – 1.5 mg,
macrogol 3350 – 500 µg,
titanium dioxide – 350 µg.
How to take, the dosage
How to take, the dosage
In order to prevent VTE in large orthopedic surgeries, it is recommended to prescribe 1 tablet 10 mg once a day.
Duration of treatment:
– 5 weeks after major hip surgery;
– 2 weeks after major knee surgery.
For the prevention of stroke and systemic thromboembolism in patients with atrial fibrillation of non-valvular origin: 15 mg and 20 mg should be taken with meals.
The recommended dose is 20 mg once daily.
Action for missed doses
If the next dose is missed, the patient should take Xarelto® immediately and continue the next day’s regular intake according to the recommended regimen.
The dose taken should not be doubled to make up for a previously missed dose.
Particular patient groups
Dose adjustments are not necessary based on patient age (>65 years), gender, body weight or ethnicity.
Patients with hepatic impairment
Xarelto® is contraindicated in patients with liver disease accompanied by coagulopathy, which causes a clinically significant risk of bleeding (see section “Contraindications”).
Patients with other liver diseases do not require dosage changes (see section “Pharmacological properties / Pharmacokinetics”).
The limited clinical data available for patients with moderate hepatic impairment indicate a significant increase in the pharmacological activity of the drug. No clinical data are available for patients with severe hepatic impairment.
Patients with impaired renal function
No dose reduction is required when prescribing Xarelto® in patients with mild to moderate renal impairment. For patients with moderate renal impairment the recommended dose is 15 mg once daily.
Limited clinical data available for patients with severe renal impairment (creatinine clearance between 30 and 15 mL/min) demonstrate a significant increase in rivaroxaban concentrations in these patients. Xarelto® should be used with caution to treat this category of patients.
The use of Xarelto® is not recommended in patients with creatinine clearance < 15 mL/min (see sections “Contraindications”, “Pharmacological properties / Pharmacokinetics”).
The switch from vitamin K antagonists (VKAs) to Xarelto®
If the INR ⤠3.0, treatment with VKAs should be discontinued and Xarelto® should be started.
If patients switch from AVC to Xarelto®, the INR will be erroneously high after taking Xarelto®. INR is not suitable for determining the anticoagulant activity of Xarelto®, and therefore should not be used for this purpose (see “Interaction with other medicinal products and other forms of interaction”).
The transition from Xarelto® to vitamin K antagonists (VKAs)
There is a possibility of insufficient anticoagulant effect when switching from Xarelto® to warfarin. Therefore, it is necessary to ensure continuous sufficient anticoagulant effect during such transition with alternative anticoagulants. It should be noted that during the transition from Xarelto® to AVC, Xarelto® may contribute to elevated INR. Thus, INR should not be used to monitor the therapeutic effect of AVC for at least 48 hours after discontinuation of Xarelto® (see “Interaction with other medicinal products and other forms of interaction”).
Transition from parenteral anticoagulants to Xarelto®
. For patients receiving parenteral anticoagulants, Xarelto® should be started 0 to 2 hours before the time of the next scheduled parenteral drug administration (e.g., low molecular weight heparin) or at the time the continuous parenteral drug administration is stopped (e.g., intravenous infractional heparin).
Transition from Xarelto® to parenteral anticoagulants
The first dose of Xarelto® should be stopped and the first dose of parenteral anticoagulant administered at the time the next dose of Xarelto® should be taken.
Interaction
Interaction
Pharmacokinetic interactions
Excretion of rivaroxaban is primarily by metabolism in the liver mediated by the cytochrome P450 system (CYP3A4, CYP2J2) and also by renal excretion of unchanged drug substance using the P-gp/Bcrp (P-glycoprotein/milk cancer resistance protein) transporter systems.
Rivaroxaban does not inhibit or induce the CYP3A4 isoenzyme or other important cytochrome isoforms.
The concomitant use of Xarelto® and potent CYP3A4 and P-glycoprotein isoenzyme inhibitors may result in decreased renal and hepatic clearance of rivaroxaban and thus significantly increase its systemic effects.
. Co-administration of Xarelto® and the azole antifungal agent ketoconazole (400 mg once daily), a potent CYP3A4 and P-glycoprotein inhibitor, resulted in a 2.6-fold increase in mean equilibrium AUC of rivaroxaban and a 1.7-fold increase in mean Cmax of rivaroxaban, with a significant increase in pharmacodynamic effects of the drug.
. Co-administration of Xarelto® and the HIV protease inhibitor ritonavir (600 mg twice daily), a potent CYP3A4 and P-glycoprotein inhibitor, resulted in a 2.5-fold increase in mean equilibrium AUC of rivaroxaban and a 1.6-fold increase in mean Cmax of rivaroxaban, with significant enhancement of the drug pharmacodynamic action. Therefore, Xarelto® is not recommended for use in patients receiving systemic treatment with azole antifungals or HIV protease inhibitors (see section “Caution”).
Clarithromycin (500 mg 2 times daily), a potent CYP3A4 isoenzyme inhibitor and moderate P-glycoprotein inhibitor, caused a 1.5-fold increase in AUC and 1.4-fold increase in Cmax values of rivaroxaban. This increase is of the order of the normal variability of AUC and Cmax and is considered clinically insignificant.
Eritromycin (500 mg 3 times daily), a moderate inhibitor of the CYP3A4 isoenzyme and P-glycoprotein, caused a 1.3-fold increase in the AUC and Cmax values of rivaroxaban. This increase is of the order of normal variability in AUC and Cmax and is considered clinically insignificant.
Fluconazole (400 mg once daily), a moderate CYP3A4 isoenzyme inhibitor, caused a 1.4-fold increase in the mean AUC of rivaroxaban and a 1.3-fold increase in mean Cmax. This increase is of the order of normal AUC and Cmax variability and is considered clinically insignificant.
Co-administration of Xarelto® and rifampicin, which is a strong inducer of CYP3A4 and P-glycoprotein, resulted in a decrease in the mean AUC of rivaroxaban by approximately 50% and a parallel decrease in its pharmacodynamic effects. Co-administration of rivaroxaban with other strong CYP3A4 inducers (e.g., phenytoin, carbamazepine, phenobarbital, or preparations of St. John’s wort) may also lead to decreased plasma concentrations of rivaroxaban. Reduced plasma concentrations of rivaroxaban have been found to be clinically insignificant. Strong CYP3A4 inducers should be used with caution.
Pharmacodynamic interactions
After concomitant use of enoxaparin sodium (single dose of 40 mg) and Xarelto® (single dose of 10 mg) the summary effect was observed in relation to activity of anti-factor Xa, without additional summary effects on clotting tests (prothrombin time, ACTV). Enoxaparin sodium did not alter the pharmacokinetics of rivaroxaban (see section “Caution”).
No pharmacokinetic interaction was found between Xarelto® (15 mg) and clopidogrel (loading dose of 300 mg followed by a maintenance dose of 75 mg), but a significant increase in bleeding time was found in a subgroup of patients that was not correlated with the degree of platelet aggregation and P-selectin or GPIIb/IIIa-receptor content (see “Cautionary Note.
No clinically significant increase in bleeding time was observed after co-administration of Xarelto® (15 mg) and naproxen at a dose of 500 mg. However, a more pronounced pharmacodynamic response is possible in individuals.
The conversion of patients from warfarin (INR 2.0 to 3.0) to Xarelto® (20 mg) increased prothrombin time/ INR (Neoplastin) to a greater extent than would be expected by simple summation of effects (individual INR values can reach 12), while effects on ACTV, suppression of factor Xa activity and endogenous thrombin potential were additive.
If the pharmacodynamic effects of Xarelto® need to be investigated during the transition period, anti-Xa activity, PiCT and HepTest® determination may be used as necessary tests that are not affected by warfarin. From day 4 after discontinuation of warfarin, all test results (including PV, ACTV, inhibition of factor Xa activity and on EPT (endogenous thrombin potential)) reflect only the effects of Xarelto® (see section “Administration and Doses”).
No pharmacokinetic interactions have been reported between warfarin and Xarelto®.
Incompatibilities
It is unknown.
Impact on laboratory parameters
The drug Xarelto® influences blood clotting parameters (PV, ACTV, HepTest®) due to its mechanism of action.
Special Instructions
Special Instructions
Antithrombotic medications, including Xarelto®, should be used with caution when treating patients with an increased risk of bleeding (see section “Caution”).
In patients with severe renal impairment, plasma concentrations of rivaroxaban may be significantly elevated and therefore at increased risk of bleeding (see Caution).
If there is an unexplained decrease in hemoglobin or blood pressure, look for the source of the bleeding.
The safety and efficacy of Xarelto® in patients with artificial heart valves has not been studied, therefore, there is no evidence that Xarelto® 20 mg (15 mg in patients with moderate renal impairment) provides sufficient anticoagulant effect in this category of patients.
In patients at risk of peptic ulcer disease, appropriate prophylactic treatment may be indicated.
If an invasive procedure or surgery is necessary, Xarelto® administration should be stopped at least 24 hours before the intervention and on the basis of a physician’s report.
If the procedure cannot be postponed, the increased risk of bleeding should be evaluated against the need for urgent intervention.
Xarelto® administration should be resumed after the invasive procedure or surgery, provided appropriate clinical signs and adequate hemostasis are present (see “Pharmacological Properties/Metabolism and Excretion” section).
Safety data from preclinical studies
With the exception of effects associated with enhanced pharmacological action (bleeding), no specific hazards to humans were found in the analysis of preclinical data from pharmacological safety studies.
The effect on the ability to drive / operate moving machinery
There have been no studies of the effect of Xarelto® on the ability to drive and operate potentially dangerous moving machinery.
In the postoperative period, fainting and dizziness have been infrequently reported (see section “Side effects”). Patients who experience these adverse reactions should not drive motor vehicles or operate moving machinery.
Contraindications
Contraindications
Hypersensitivity to rivaroxaban or any excipients contained in Xarelto tablet.
Clinically significant active bleeding (e.g., intracranial bleeding, gastrointestinal bleeding).
Liver diseases occurring with coagulopathy, which causes clinically significant risk of bleeding.
Pregnancy and lactation (period of breast-feeding).
Children and adolescents under 18 years of age (efficacy and safety of Xarelto for patients in this age group has not been established).
The use of rivaroxaban has not been studied in clinical trials in surgical interventions in patients with femoral fractures. Therefore, the use of rivaroxaban is not recommended for this category of patients.
There are no clinical data on the use of rivaroxaban in patients with severe renal impairment (creatinine clearance < 15 ml/min). Therefore, the use of rivaroxaban is not recommended for this category of patients.
With caution. When treating patients with an increased risk of bleeding (including congenital or acquired tendency to bleeding, uncontrolled severe arterial hypertension, acute gastric and duodenal ulcer Recent gastric and duodenal ulcer disease, vascular retinopathy, recent intracranial or intracerebral hemorrhage, pathology of spinal or cerebral blood vessels, after recent surgery on the brain, spinal cord, or eyes).
– In the treatment of patients with renal insufficiency of moderate severity (creatinine clearance between 49-30 ml/min) receiving simultaneously the drugs that increase the concentration of rivaroxaban in plasma.
– Caution should be exercised when treating patients with severe renal insufficiency (creatinine clearance between 29-15 mL/min) because such patients are at increased risk of both bleeding and thrombosis due to the underlying disease.
– Rivaroxaban is not recommended for use in patients receiving systemic treatment with azole antifungals (e.g., ketoconazole) or HIV protease inhibitors (e.g., ritonavir). These drugs are strong inhibitors of CYP3A4 isoenzyme and P-glycoprotein. As a consequence, these drugs may increase the plasma concentration of rivaroxaban to clinically significant levels, which increases the risk of bleeding (see section “Interaction with other medicinal products”).
Patients with severe renal impairment or increased risk of bleeding and patients receiving concomitant systemic treatment with azole antifungals or HIV protease inhibitors should be monitored closely after treatment initiation for timely detection of bleeding complications. Such monitoring may include regular physical examination of patients, close monitoring of surgical wound drainage, and periodic measurement of hemoglobin levels. Any decrease in hemoglobin or blood pressure for which there is no explanation is grounds for looking for the site of bleeding.
– In patients receiving medications that affect hemostasis (e.g., NSAIDs, antiaggregants or other antithrombotic agents).
– Because this medication contains lactose, patients with hereditary lactose or galactose intolerance (e.g., caused by Lapp lactase deficiency or glucose-galactose malabsorption) should not take rivaroxaban.
Side effects
Side effects
The safety of Xarelto® 10 mg has been evaluated in four phase III studies involving 6097 patients who underwent major orthopedic lower extremity surgery (total knee or hip replacement) and were treated with Xarelto® for up to 39 days and in two phase III studies of venous thromboembolism including 2194 patients receiving either 15 mg Xarelto® 2 times/day daily for 3 weeks, followed by a dose of 20 mg once daily or 20 mg once daily for up to 21 months.
In addition, safety data in patients with non-valvular atrial fibrillation were obtained in two phase III studies involving 7,750 patients who received at least one dose of Xarelto®.
With the mechanism of action, use of Xarelto® may be accompanied by an increased risk of occult or overt bleeding from any organ or tissue, which may lead to post-hemorrhagic anemia. The risk of bleeding may increase in patients with uncontrolled arterial hypertension and/or when co-administered with drugs that affect hemostasis.
The signs, symptoms and severity (including lethality) vary depending on the location, intensity or duration of bleeding and/or anemia.
Hemorrhagic complications may manifest as weakness, pallor, dizziness, headache, shortness of breath, and limb enlargement or shock that cannot be explained by other causes. In some cases, symptoms of myocardial ischemia, such as chest pain and angina, have been observed due to anemia.
Known complications secondary to severe bleeding, such as interfacial space syndrome and renal failure, have been reported while taking Xarelto®. Therefore, the possibility of hemorrhage should be considered when evaluating a patient receiving anticoagulants.
The frequency of adverse reactions reported for Xarelto® is summarized below. In groups divided by frequency, adverse effects are presented in decreasing order of severity as follows: very frequently (â¥1/10), frequently (â¥1/100 and < 1/10), infrequently (â¥1/1000 and < 1/100), rarely (â¥1/10 000 and < 1/1000), very rarely (< 1/10 000).
Hematopoietic system disorders: frequent – anemia (including relevant laboratory parameters); infrequent – thrombocythemia (including increased platelet count)*.
Cardiovascular system disorders: frequently – tachycardia, hypotension, hematoma.
Visually: often – ocular hemorrhage (including conjunctival hemorrhage).
Gastrointestinal system: frequently – gastrointestinal bleeding (including gum bleeding and rectal bleeding), abdominal pain, dyspepsia, nausea, constipation*, diarrhea, vomiting*; infrequently – dry mouth, liver function impairment; rarely – jaundice.
Laboratory tests: frequently – increased transaminase levels; infrequent – increased bilirubin concentration, increased alkaline phosphatase activity*, increased LDH activity*, increased lipase activity*, increased amylase activity*, increased GGT level*; rarely – increased concentration of conjugated bilirubin (with or without accompanying elevated ALT activity).
CNS disorders: frequently – dizziness, headache, syncope; infrequent – intracerebral and intracranial hemorrhage.
Urogenital system disorders: common – bleeding from the urinary tract (including hematuria and menorrhagia**); infrequent – renal failure (including increased creatinine concentration, increased concentration of urea)*.
Respiratory system disorders: frequently – nasal bleeding: infrequently – hemoptysis.
The skin: often – itching (including rare cases of generalized itching), rash, ecchymosis; infrequently – urticaria, cutaneous and subcutaneous hemorrhage.
Immune system disorders: infrequent allergic reactions, allergic dermatitis.
Muscular system: often – pain in the extremities *; infrequent – hemarthrosis; rarely – bleeding into the muscles.
Actually, fever*, peripheral edema, malaise (including discomfort), local edema.
Others: often – hemorrhage after procedures (including postoperative anemia and wound bleeding), post-traumatic hematoma; infrequent – discharge from the wound*.
* – reported after major orthopedic surgery.
** – reported in VTE treatment as very frequent in women <55 years.
Other clinical studies have reported the occurrence of vascular pseudoaneurysm after percutaneous interventions.
Overdose
Overdose
Rivalroxaban overdose may lead to hemorrhagic complications due to the pharmacodynamic properties of the drug.
The specific antidote for rivaroxaban is unknown.
In case of overdose, activated charcoal may be used to reduce absorption of rivaroxaban. Taking activated charcoal within 8 hours of an overdose can reduce the absorption of rivaroxaban.
Given the intense binding to plasma proteins, rivaroxaban is not expected to be excreted by dialysis.
If bleeding occurs, the following steps may be taken to control it:
– Later administration of the next dose of rivaroxaban or withdrawal of treatment, as appropriate. The half-life of rivaroxaban is approximately 5 to 13 hours (see Pharmacokinetics).
– Appropriate symptomatic treatment should be considered, such as mechanical compression (e.g. in cases of severe nasal bleeding), surgical intervention, fluid replacement and hemodynamic support, blood transfusion or blood components.
If the above measures do not stop bleeding, one of the following procoagulants may be prescribed:
– activated prothrombin complex concentrate (APCC)
– prothrombin complex concentrate (RCC)
– recombinant factor VIIa (rf VIIa).
However, to date, there is no experience with these products in the treatment of patients receiving rivaroxaban.
Protamine sulfate and vitamin K are not expected to affect the anticoagulation activity of rivaroxaban. There is no scientific rationale or experience with the use of systemic hemostatic agents (e.g., desmopresin, aprotinin, tranexamic acid, aminocaproic acid) to reverse rivaroxaban overdose.
Pregnancy use
Pregnancy use
Pregnancy
There are no data on the use of rivaroxaban in pregnant women.
The data obtained on experimental animals showed a pronounced maternal toxicity of rivaroxaban associated with the pharmacological action of the drug (e.g., complications in the form of hemorrhage) and leading to reproductive toxicity.
Due to possible risk of bleeding and ability to penetrate through the placenta rivaroxaban is contraindicated in pregnancy.
Women with preserved fertility should use effective contraception during treatment with rivaroxaban.
Lactation
There are no data on the use of rivaroxaban for the treatment of women during lactation. Data obtained in experimental animals show that rivaroxaban is excreted with breast milk. Rivaroxaban can be used only after cancellation of
breastfeeding.
Fertility
At doses up to 200 mg/kg, rivaroxaban has no effect on male or female fertility.
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | At a temperature not exceeding 30 °C |
| Manufacturer | Bayer AG, Germany |
| Medication form | pills |
| Brand | Bayer AG |
Other forms…
Related products
Buy Xarelto, 15 mg 100 pcs with delivery to USA, UK, Europe and over 120 other countries.