No products in the cart.
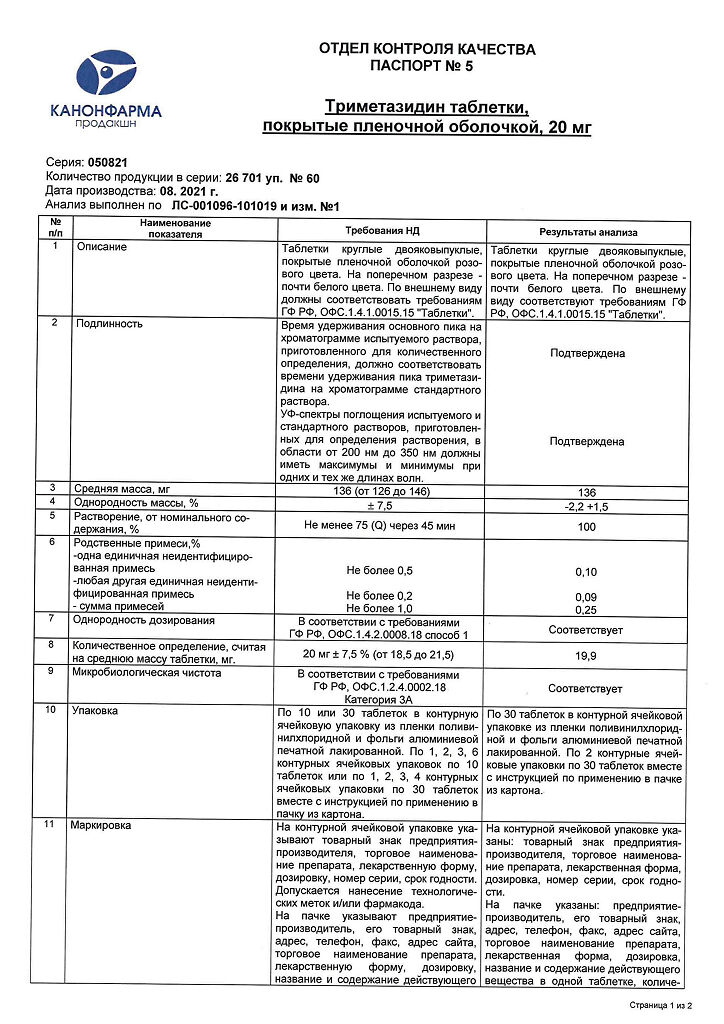
Trimetazidine, 20 mg 60 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
Pharmacological action – antianginal, antihypoxic, cytoprotective, metabolic.
Directly affects cardiomyocytes and neurons of the brain, optimizing their metabolism and functions. Cytoprotection is due to sufficient energy potential, activation of oxidative decarboxylation and rationalization of oxygen consumption (increase of aerobic glycolysis and blockade of fatty acid oxidation). Supports myocardial contractility, prevents intracellular depletion of ATP and phosphocreatinine. Under acidosis, it normalizes the functioning of membrane ion channels, prevents the accumulation of calcium and sodium in cardiomyocytes and normalizes intracellular potassium content.
Limits intracellular acidosis and phosphate content caused by myocardial ischemia and reperfusion. Prevents damaging action of free radicals, maintains integrity of cell membranes, prevents activation of neutrophils in ischemic area, increases duration of electric potential, decreases release of creatine phosphokinase from cells and intensity of ischemic myocardial damage.
In angina pectoris it reduces the frequency of attacks (nitrates consumption decreases), after 2 weeks of treatment the tolerance to physical load increases, BP fluctuations decrease.
It improves hearing and results of vestibular tests in patients with ENT pathology, reduces dizziness and tinnitus. In case of vascular eye pathology it restores the functional activity of the retina.
It is completely and quickly absorbed from the gastrointestinal tract, the bioavailability is 90%. Tmax – 2 hours. Cmax after single oral administration of 20 mg trimetazidine is 55 ng/ml. Binding to plasma proteins is 16%. The volume of distribution is 4.8 l/kg. Easily passes through histohematic barriers. Almost half of the administered amount undergoes biotransformation. T1/2 is about 6 hours. 51% is excreted unchanged by the kidneys.
Indications
Indications
Chorioretinal vascular disorders
Dizziness of vascular origin
Coronary heart disease: prevention of angina attacks (in complex therapy):
Cochleo-vestibular disorders of ischemic nature (tinnitus, hearing impairment).
Pharmacological effect
Pharmacological effect
Pharmacological action – antianginal, antihypoxic, cytoprotective, metabolic.
Directly affects cardiomyocytes and neurons of the brain, optimizing their metabolism and functions. Cytoprotection is due to the provision of sufficient energy potential, activation of oxidative decarboxylation and rationalization of oxygen consumption (increased aerobic glycolysis and blockade of fatty acid oxidation). Supports myocardial contractility, prevents intracellular depletion of ATP and phosphocreatinine. In conditions of acidosis, it normalizes the functioning of membrane ion channels, prevents the accumulation of calcium and sodium in cardiomyocytes, and normalizes the intracellular potassium content.
Reduces intracellular acidosis and phosphate content caused by myocardial ischemia and reperfusion. Prevents the damaging effects of free radicals, preserves the integrity of cell membranes, prevents activation of neutrophils in the ischemic zone, increases the duration of the electrical potential, reduces the release of creatine phosphokinase from cells and the severity of ischemic damage to the myocardium.
In case of angina pectoris, it reduces the frequency of attacks (nitrate consumption decreases), after 2 weeks of treatment, exercise tolerance increases, and blood pressure changes decrease.
Improves hearing and the results of vestibular tests in patients with ENT pathologies, reduces dizziness and tinnitus. In case of vascular pathology of the eye, it restores the functional activity of the retina.
Completely and quickly absorbed from the gastrointestinal tract, bioavailability is 90%. Tmax – 2 hours. Cmax after a single oral dose of 20 mg trimetazidine is 55 ng/ml. Plasma protein binding – 16%. Volume of distribution – 4.8 l/kg. Easily passes through histohematic barriers. Almost half of the administered amount undergoes biotransformation. T1/2 is about 6 hours. 51% is excreted unchanged by the kidneys.
Special instructions
Special instructions
Do not use to relieve angina attacks. Not indicated for the initial course of treatment of unstable angina or myocardial infarction. If an attack of angina occurs, treatment should be reviewed and adapted.
Impact on driving vehicles and machinery. Trimetazidine does not affect the ability to drive vehicles and perform work that requires an increased speed of psychomotor reactions.
Active ingredient
Active ingredient
Trimetazidine
Composition
Composition
1 film-coated tablet contains:
active ingredient:
trimetazidine dihydrochloride – 20 mg
excipients:
hyprolose (hydroxypropylcellulose) 0.7 mg,
corn starch 16 mg,
colloidal silicon dioxide (aerosil) 3.13 mg,
lactose monohydrate (milk sugar) 89.47 mg,
magnesium stearate 0.7 mg.
film shell composition:
selecoate AQ-01673 – 6 mg: hypromellose (hydroxypropylmethylcellulose) 2.4 mg, macrogol-400 (polyethylene glycol-400) 0.6 mg, macrogol-6000 (polyethylene glycol-6000) 0.6 mg, crimson dye [Ponceau 4 R] 0.6 mg, titanium dioxide 1.8 mg
Pregnancy
Pregnancy
Trimetazidine is contraindicated during pregnancy due to the lack of clinical data on the safety of its use. If it is necessary to prescribe the drug during lactation, breastfeeding should be stopped.
It is not known whether trimetazidine is excreted in breast milk.
Experimental studies have not established the teratogenic effect of trimetazidine.
Contraindications
Contraindications
Hypersensitivity to any component of Trimetazidine;
Severe liver dysfunction;
Renal failure (creatinine clearance below 15 ml/min);
Pregnancy;
Breastfeeding period:
Age up to 18 years (efficacy and safety have not been established).
Side Effects
Side Effects
The frequency of side effects noted when taking trimetazidine is given in the following gradation: very often (more than 1/10); often (more than 1/100, less than 1/10); uncommon (more than 1/1000, less than 1/100); rare (more than 1/10000, less than 1/1000); very rare (less than 1/10000), including isolated messages.
From the digestive system:
Common: abdominal pain, diarrhea, dyspepsia, nausea, vomiting.
General violations:
Often: asthenia.
From the central nervous system:
Common: dizziness, headache.
Very rare: extrapyramidal disorders (tremor, rigidity, akinesia), reversible after discontinuation of the drug.
From the skin:
Common: skin rash, itching, urticaria.
From the cardiovascular system:
Rarely: orthostatic hypotension, “flushes” of blood to the facial skin.
Interaction
Interaction
No information available.
Overdose
Overdose
Data on overdose cases are limited. In case of overdose, symptomatic therapy should be carried out.
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25 °C
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Kanonpharma production CJSC, Russia
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature not exceeding 25 °C |
| Manufacturer | Kanonfarma Production ZAO, Russia |
| Medication form | pills |
| Brand | Kanonfarma Production ZAO |
Other forms…
Related products
Buy Trimetazidine, 20 mg 60 pcs with delivery to USA, UK, Europe and over 120 other countries.