No products in the cart.
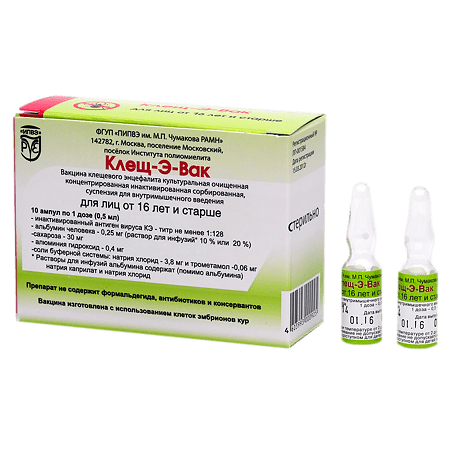
Tick-E-Vac, 0.5 ml/dose 0.5 ml 10 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
Specific prophylaxis of tick-borne encephalitis for persons aged 16 years and older in a dose of 0.5 ml and for children from 1 to 16 years in a dose of 0.25 ml;
The immunization of donors in order to obtain specific immunoglobulin.
Contingents subject to specific prophylaxis:
1. Population living in areas enzootic for tick-borne encephalitis.
2. Persons arriving in these areas who perform the following work:
– agricultural, hydromelioration, construction, excavation and relocation of soil, procurement, fishing, geological, surveying, expeditionary, deratization and disinfestation.
– on logging, clearing and improvement of forests, recreation and recreation areas.
3. Persons visiting areas endemic for tick-borne encephalitis for recreation, tourism, work in summer cottages and garden plots.
4 Persons working with live cultures of tick-borne encephalitis pathogen.
Indications
Indications
– Specific prevention of tick-borne encephalitis for persons aged 16 years and older at a dose of 0.5 ml and for children from 1 year to 16 years at a dose of 0.25 ml;
– immunization of donors in order to obtain specific immunoglobulin.
Contingents subject to specific prevention:
1. Population living in areas enzootic for tick-borne encephalitis.
2. Persons arriving in these territories performing the following work:
– agricultural, drainage, construction, excavation and movement of soil, procurement, fishing, geological, survey, expedition, deratization and disinfestation.
– logging, clearing and landscaping of forests, health and recreation areas for the population.
3. Persons visiting areas endemic for tick-borne encephalitis for the purpose of recreation, tourism, work in summer cottages and gardens.
4. Persons working with live cultures of the causative agent of tick-borne encephalitis.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group:
Special instructions
Special instructions
Vaccinations are carried out in strict compliance with the rules of asepsis and antiseptics. The room must be equipped with anti-shock and anti-allergic therapy.
Before opening the ampoule, it is necessary to carry out a visual inspection. The drug is not suitable in ampoules with damaged integrity, labeling, if foreign inclusions are detected, if there are large unbreakable conglomerates, if the expiration date has expired, if the temperature conditions of storage or transportation are violated.
Immediately before injection, the vaccine in the ampoule is shaken until a homogeneous suspension is obtained. The drug is administered immediately after opening the ampoule intramuscularly into the deltoid muscle of the shoulder.
Vaccinations performed are recorded in established registration forms indicating the name of the drug, date of vaccination, dose, batch number, manufacturer, reaction to vaccination.
The drug cannot be administered intravenously!
Vaccination of children and adults with chronic diseases in the acute stage is carried out no earlier than 1 month after recovery (remission).
The vaccine is not used for children under 1 year of age.
Impact on the ability to drive vehicles. Wed and fur.:
Severe general reactions to the vaccine (significant increase in temperature, severe headache) are a contraindication for driving vehicles and machinery.
Active ingredient
Active ingredient
Vaccine for the prevention of tick-borne encephalitis
Composition
Composition
One vaccination dose for persons aged 16 years and older (0.5 ml) contains:
Contraindications
Contraindications
1. Acute infectious and non-infectious diseases, chronic diseases in the acute stage – vaccinations are carried out no earlier than 1 month after recovery (remission);
2. History of severe allergic reactions; bronchial asthma; autoimmune diseases.
3. History of allergy to the components of the drug.
4. Severe reaction (fever above 40 °C; swelling at the site of vaccine administration, hyperemia more than 8 cm in diameter) or complications to the previous dose of the vaccine.
5. Children under 1 year.
When vaccinating donors, the contraindications listed above, as well as contraindications related to donor selection, should be taken into account.
In each case of a disease not contained in this list of contraindications, vaccination is carried out with the permission of a doctor, based on the health status of the person being vaccinated and the risk of infection with tick-borne encephalitis. In order to identify contraindications, the doctor (paramedic) conducts a survey and examination of the vaccinated person on the day of vaccination with mandatory thermometry.
Side Effects
Side Effects
After administration of the vaccine, local and general reactions may develop in some cases.
When assessing adverse drug reactions, the following frequency data were used as the basis:
very often >10%;
often – from 1 to 10%;
from case to case – from 0.1 to 1%;
rarely – from 0.01 to 0.1%;
very rare – <0.01%, including isolated cases.
Interaction
Interaction
It is allowed to vaccinate against tick-borne encephalitis simultaneously (on the same day) with other vaccinations with inactivated vaccines of the National Preventive Vaccination Calendar and the Preventive Vaccination Calendar for epidemic indications (with the exception of rabies).
Overdose
Overdose
No cases of overdose have been identified.
Storage conditions
Storage conditions
The drug is stored and transported at a temperature of 2 to 8 °C.
Shelf life
Shelf life
2 years.
A drug that has expired cannot be used.
Manufacturer
Manufacturer
FSUE Institute named after Chumakov, Russia
Additional information
| Shelf life | 2 years.Preparation with expired shelf life cannot be used. |
|---|---|
| Conditions of storage | The drug is stored and transported at a temperature of 2 to 8 ° C. |
| Manufacturer | FSUE Chumakov Institute, Russia |
| Medication form | suspension |
| Brand | FSUE Chumakov Institute |
Related products
Buy Tick-E-Vac, 0.5 ml/dose 0.5 ml 10 pcs with delivery to USA, UK, Europe and over 120 other countries.