No products in the cart.
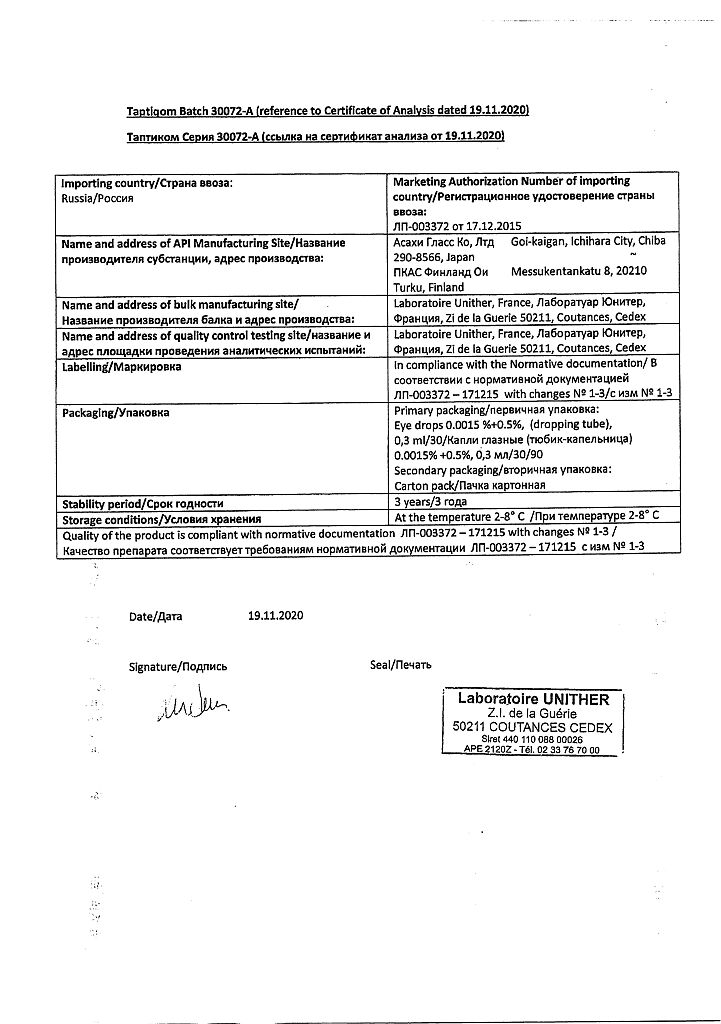
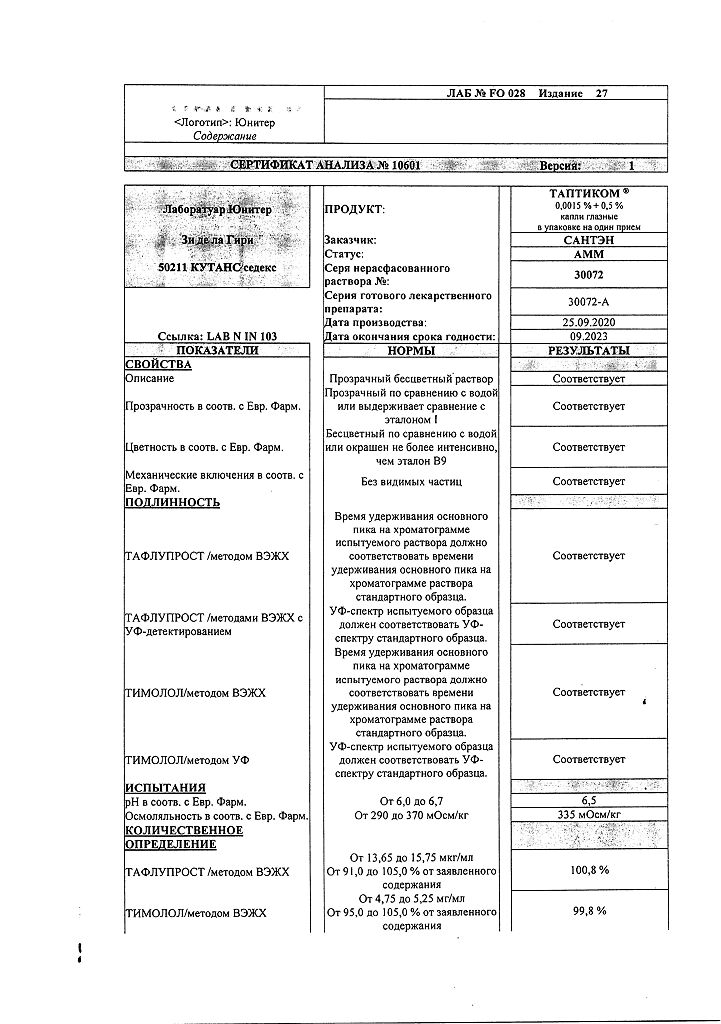
Tapticom, eye drops 0.0015%+0.5% 0.3 ml tube-caps 30 pcs
€38.26 €31.89
Description
Pharmacodynamics.
The mechanism of action
Tapticom® is a combination medication containing two active ingredients, tafluprost and timolol. The two active ingredients reduce intraocular pressure (IOP) through additional mechanisms of action, and the combined action results in an additional reduction in IOP compared to the action of either substance alone.
Tafluprost is a fluorinated analog of prostaglandin F 2ɑ . Tafluprost acid, the biologically active metabolite of tafluprost, is a highly active selective antagonist of the human prostaglandin FP receptor.
Pharmacodynamic studies in animals show that tafluprost reduces intraocular pressure by increasing uveoscleral fluid outflow in the eye.
Timolol maleate is a non-selective beta-adrenoreceptor. The exact mechanism of action of timolol maleate in reducing intraocular pressure has not yet been fully established, although fluorescein and tonography studies suggest that the main action may be related to reduced fluid production. However, some studies have also shown a slight increase in outflow activation.
Clinical efficacy
In a 6-month study (n = 400) of patients with open-angle glaucoma or ophthalmohypertension and a mean untreated IOP of 24 to 26 mmHg.The IOP-lowering effect of Tuptic® (once daily in the morning) was compared with the combined administration of 0.0015% tafluprost (once daily in the morning) and 0.5% timolol (twice daily). Tapticom® was no less effective (not inferior) than 0.0015% tafluprost and 0.5% timolol, which were used together. The mean daily decrease in WTO relative to initial values was 8 mmHg in both groups with a primary endpoint of 6 months (decreases
were in the range of 7 to 9 mmHg in both groups at different times during the day during the study visits).
In another 6-month study (n = 564), Tuptic® was compared with appropriate monotherapy in patients with open-angle glaucoma or ophthalmohypertension and a mean untreated WTO of 26 to 27 mm Hg. Patients insufficiently controlled with 0.0015% tafluprost (WTO 20 mm Hg or greater with treatment) or 0.5% timolol (WTO 22 mm Hg or greater with treatment) were randomized to treatment with Taptic® or the same monotherapy. The mean daily reduction in WTO with Tapticom® was statistically greater than the mean daily reduction in WTO with tafluprost, which patients received once daily in the morning, or with timolol, which they received twice daily, at visits at 6 weeks, at 3 months (the primary endpoint of effectiveness assessment), and at 6 months. The mean daily reduction in WTO relative to baseline values was 9 mmHg when taking Tapticom® compared with 7 mmHg when taking both monotherapies. The decrease in HF when taking Tapticom® at different times during the day ranged from 8 to 9 mmHg in the comparison group, used tafluprost as monotherapy, and from 7 to 9 mmHg in the comparison group, used timolol as monotherapy.
Summarized data from patients who took Tapticom® and had a high initial WTO of 26 mm Hg (mean daily) or higher in the two baseline studies (n = 168) showed a mean daily reduction in WTO of 10 mm Hg at the primary endpoint (3 or 6 months), ranging from 9 to 12 mm Hg at various times during the day.
pharmacokinetic properties
absorption
. Plasma concentrations of tafluprostoic acid and timolol were examined in healthy volunteers after a single and repeated administration of Tapticom® for eight days (once daily), 0.0015% tafluprost (once daily) and 0.5% timolol (twice daily).The plasma concentration of tafluprostic acid reached its maximum value 10 minutes after the dose and decreased below the lower limit of detection (10 pg/mL) 30 minutes after administration of Tapticom® . The accumulation of tafluprostic acid was insignificant, and the mean urinary concentration of tafluprostic acid (mean AUC 0- last ) (monotherapy: 4.45 + 2.57 pg – h / ml Taptic® : 3.60 + 3.70 pg – h / mL) and mean maximum concentration ( max ) (monotherapy: 23.9 +11.8 pg – mL Taptic® : 18.7 + 11.9 pg – mL) were slightly lower with treatment with Taptic® compared to day 8 monotherapy with tafluprost. Plasma thymolol concentration reached its maximum value at a median T max (median time to reach maximum drug concentration) of 15 and 37.5 minutes after administration of Tuptic® on days 1 and 8, respectively. On day 8, the mean urinary thymolol concentration (mean AUC 0- last ) (monotherapy 5750 + 2440 pg – h / ml Tapticom® 4560 + 2980 pg – h / ml) and mean maximum concentration ( max ) (monotherapy: 1100 + 550 pg / ml Tapticom® 840 + 520 pg / ml) were slightly lower with treatment with Tapticom® compared with thymololol monotherapy. The lower plasma thymolol levels with treatment with Tuptic® are probably related to the once-daily dose of Tuptic® compared to the twice-daily dose of thymolol as monotherapy.
Tafluprost and timolol are absorbed through the cornea. In animals, after a single injection, corneal penetration of tafluprost with Tapticom® was similar to that with tafluprost as monotherapy, while timolol penetration was slightly less with Tapticom® compared to that with timolol as monotherapy. As for tafluprostic acid, the 4 h AUC was 7.5 ng – h / ml after administration of Tapticom® and 7.7 ng – h / ml after administration of tafluprost as monotherapy. For timolol, the 4 h AUC was 585 ng – h / mL and 737 ng – h / mL after administration of Tapticom® and timolol monodrug, respectively. The T max for tafluprostic acid was 60 minutes for both Taptic® and the monotherapy with tafluprost, whereas the T max for timolol was 60 minutes for Taptic® and 30 minutes for timolol monotherapy.
The distribution
Tafluprost
Tafluprost. There was no specific distribution of radioactive isotope-labeled tafluprost in the iridium-ciliary zone or vasculature (chorioid) or retinal pigment epithelium in animals, indicating low melanin pigment affinity. In a study with total actoradiography in animals, the highest concentration of radioactivity was observed in the cornea, and then in the eyelids, sclera, and iris. Outside the eye, radioactivity spread to the lacrimal organs, palate, esophagus and gastrointestinal tract, kidneys, liver, gallbladder, and bladder. The in vitro binding of tafluprostic acid to human serum albumin was 99% at a tafluprostic acid concentration of 500 ng/mL.
Timolol
In animals, the maximum level of timolol-labeled radioactivity in the ocular fluid was reached 30 minutes after a single injection of timolol labeled with the radioactive isotope 3 H (0.5% solution: 20 µl/eye) into both eyes. Thymolol is removed from the ocular fluid much faster than from the pigmented iris and ciliary body tissues.
Metabolism
Tafluprost
The major metabolic pathway of tafluprost in humans that has been studied in vitro is hydrolysis to the pharmacologically active metabolite, tafluprostoic acid, which is further metabolized by glucuronidation or beta-oxidation. The beta-oxidation products 1,2-dinor and 1,2,3,4-tetranor of tafluprostic acid, pharmacologically inactive, may be subject to glucuronidation or hydroxylation. The cytochrome P450 (CYP) enzyme system is not involved in the metabolism of tafluprostic acid. According to the results of studies of animal corneal tissue with purified enzymes, the main esterase responsible for esterase hydrolysis to tafluprostic acid is carboxylesterase. Butylcholinesterase, but not acetylcholinesterase, can also contribute to hydrolysis.
Timolol
Timolol is metabolized in the liver, mainly by the enzyme CYP2D6, to inactive metabolites, which are eliminated mainly by the kidneys.
Exhaustion
Tafluprost
. After tafluprost, labeled with the radioactive isotope 3 H (0.005% ophthalmic solution, 5 µl/eye), was injected once daily for 21 days into both eyes in animals, approximately 87% of the total radioactive dose was excreted. The total amount that was excreted with urine was about 27-38% of the dose and about 44-58% of the dose was excreted with feces.
Timolol
The established plasma elimination half-life is approximately 4:00. Timolol is actively metabolized in the liver; metabolites are excreted in the urine along with 20% in unchanged timolol after oral administration.
Indications
Indications
Reduction of intraocular pressure (IOP) in adult patients with open-angle glaucoma or ocular hypertension who do not respond adequately to topical monotherapy with beta-blockers or prostaglandin analogues and require combination therapy and who are indicated for the use of preservative-free eye drops.
Pharmacological effect
Pharmacological effect
Pharmacodynamics.
mechanism of action
Tapticom ® is a combination drug containing two active ingredients – tafluprost and timolol. These two active substances reduce intraocular pressure (IOP) through complementary mechanisms of action, and the combined action results in an additional reduction in IOP compared with the action of only one of the two substances.
Tafluprost is a fluorinated analogue of prostaglandin F2ɑ. Tafluprost acid, a biologically active metabolite of tafluprost, is a highly active selective antagonist of the human prostanoid FP receptor.
Pharmacodynamic studies in animals show that tafluprost reduces intraocular pressure by increasing uveoscleral outflow of ocular fluid.
Timolol maleate is a non-selective beta-adrenergic receptor. The exact mechanism of action of timolol maleate in lowering intraocular pressure is not yet fully established, although fluorescein studies and tonography studies suggest that the main effect may be related to reduced fluid formation. However, some studies also observed a slight increase in outflow activation.
clinical effectiveness
In a 6-month study (n = 400) of patients with open-angle glaucoma or ocular hypertension and an average untreated VTO of 24 to 26 mmHg. The IOP-lowering effect of Taptiq ® (once daily in the morning) was compared with co-administration of 0.0015% tafluprost (once daily in the morning) and 0.5% timolol (twice daily). Tapticom ® was no less effective (not inferior) in its effects than 0.0015% tafluprost and 0.5% timolol, which were used together. The average daily decrease in VTO relative to the initial values was 8 mm Hg. Art. in both groups with a primary endpoint of 6 months (reduction
ranged from 7 to 9 mm Hg. Art. in both groups at different time points throughout the day during the study visits).
In another 6-month study (n = 564), Taptiq ® was compared with corresponding monotherapy in patients with open-angle glaucoma or ocular hypertension and an average untreated VTO of 26 to 27 mmHg. Art. Patients inadequately controlled with 0.0015% tafluprost (HTO 20 mm Hg or more on treatment) or 0.5% timolol (HTO 22 mm Hg or more on treatment) were randomized to treatment with Taptiq ® or the same monotherapy. The average daily reduction in TTO with Tapticom ® was statistically greater than the average daily decrease in TTO when taking tafluprost, which patients received once daily in the morning, or when taking timolol, which they received twice daily, at the 6-week, 3-month (primary efficacy endpoint) and 6-month visits. The average daily decrease in VTO relative to the initial values when taking the drug Tapticom ® was 9 mm Hg. Art. compared to 7 mm Hg. Art., which was observed when taking both monotherapies. The decrease in VTO when taking the drug Tapticom ® at various times during the day ranged from 8 to 9 mm Hg. Art. in the comparison group, used tafluprost as monotherapy, and from 7 to 9 mm Hg. Art. in the comparison group, timolol was used as monotherapy.
Summary data obtained from patients who took Taptiq ® and had a high initial VTO of 26 mmHg. Art. (average daily) or higher in two pivotal studies (n = 168), showed that the average daily decrease in VTO was 10 mm Hg. Art. at the primary endpoint (3 or 6 months), ranging from 9 to 12 mm Hg. Art. at various times during the day.
pharmacokinetic properties
absorption
Plasma concentrations of tafluprost acid and timolol were studied in healthy volunteers after single and repeated doses of Tapticom ® for eight days (once daily), 0.0015% tafluprost (once daily) and 0.5% timolol (twice daily). Plasma concentrations of tafluprost acid reached a maximum value 10 minutes after dosing and decreased below the lower limit of detection. (10 pg/ml) 30 minutes after taking the drug Tapticom ®. The accumulation of tafluprost acid was insignificant, and the mean concentration of tafluprost acid in urine (mean AUC 0-last) (monotherapy: 4.45 + 2.57 pg • h / ml Tapticom ®: 3.60 + 3.70 pg • h / ml) and the mean maximum concentration (max) (monotherapy: 23.9 + 11.8 pg / ml Tapticom ® : 18.7 + 11.9 pg/ml) were slightly lower during treatment with Tapticom ® compared to tafluprost monotherapy on day 8. The concentration of timolol in the blood plasma reached its maximum value at a median Tmax (median time to reach maximum drug concentration) of 15 and 37.5 minutes after taking Tapticom ® on days 1 and 8, respectively. On day 8, the mean concentration of timolol in urine (mean AUC 0-last) (monotherapy 5750 + 2440 pg • h / ml Tapticom ® 4560 + 2980 pg • h / ml) and the mean maximum concentration (max) (monotherapy: 1100 + 550 pg / ml Tapticom ® 840 + 520 pg / ml) were slightly lower when treated with Taptiq ® compared to timolol monotherapy. The low levels of timolol in plasma during treatment with Taptiq ® are likely due to the once-daily dosing of Taptiq ® compared to the twice-daily dosing of timolol as monotherapy.
Tafluprost and timolol are absorbed through the cornea. In animals, after a single instillation, the penetration of tafluprost through the cornea when using the drug Tapticom ® was similar to that when using tafluprost as monotherapy, while the penetration of timolol was slightly less when using the drug Tapticom ® compared to that when using timolol as a monotherapy. As for tafluprost acid, the AUC 4 h was 7.5 ng • h / ml after taking Tapticom ® and 7.7 ng • h / ml after taking tafluprost as a single drug. For timolol, AUC 4 h was 585 ng • h / ml and 737 ng • h / ml after taking Tapticom ® and timolol alone, respectively. The T max for tafluprost acid was 60 minutes for both treatment with Taptiq ® and the treatment with tafluprost monotherapy, while the T max for timolol was 60 minutes for treatment with Taptiq ® and 30 minutes for timolol monotherapy.
distribution
Tafluprost
The animals did not have a specific distribution of radiolabeled tafluprost in the iridium-ciliary zone or choroid, nor in the retinal pigment epithelium, indicating low affinity of the melanin pigment. In a general actoradiography study in animals, the highest concentrations of radioactivity were observed in the cornea, followed by the eyelids, sclera, and iris. Outside the eye, radioactivity spread to the lacrimal organs, palate, esophagus and gastrointestinal tract, kidneys, liver, gall bladder and bladder. The binding of tafluprost acid to human serum albumin in vitro was 99% at a tafluprost acid concentration of 500 ng/ml.
timolol
In animals, the maximum level of timolol-associated radioactivity in the ocular fluid was achieved 30 minutes after a single instillation of 3H radiolabeled timolol (0.5% solution: 20 μl/eye) into both eyes. Timolol is removed from the fluid of the eye much faster than from the tissues of the pigmented iris and ciliary body.
Metabolism
Tafluprost
The main metabolic pathway of tafluprost in humans, which has been studied in vitro, is hydrolysis to the pharmacologically active metabolite, tafluprost acid, which is further metabolized by glucuronidation or beta-oxidation. The beta-oxidation products of 1,2-dinor and 1,2,3,4-tetranor tafluprost acid, which are pharmacologically inactive, may be subject to glucuronidation or hydroxylation. The cytochrome P450 (CYP) enzyme system is not involved in the metabolism of tafluprost acid. Based on the results of studies of animal corneal tissue with purified enzymes, the main esterase responsible for ester hydrolysis to tafluprost acid is carboxylesterase. Butylcholinesterase, but not acetylcholinesterase, may also promote hydrolysis.
timolol
Timolol is metabolized in the liver, mainly by the enzyme CYP2D6 to inactive metabolites, which are excreted primarily by the kidneys.
Removal
Tafluprost
Following instillation of 3H radiolabeled tafluprost (0.005% ophthalmic solution, 5 µl/eye) once daily for 21 days in both eyes of animals, approximately 87% of the total radioactive dose was excreted. The total amount excreted in the urine was approximately 27-38% of the dose and approximately 44-58% of the dose was excreted in the feces.
timolol
The estimated plasma half-life is approximately 4:00. Timolol is actively metabolized in the liver, metabolites are excreted from the body in the urine along with 20% of unchanged timolol after oral administration.
Active ingredient
Active ingredient
Tafluprost, Timolol
Composition
Composition
1 ml of eye drops contains 0.015 mg of tafluprost and 5 mg of timolol, which corresponds to 6.84 mg of timolol maleate;
excipients:
glycerin sodium phosphate,
dodecahydrate;
Trilon B,
polysorbate 80,
sodium hydroxide and/or concentrated hydrochloric acid,
water for injections.
Contraindications
Contraindications
Allergic reactions to the active substances or to any of the auxiliary components of the drug.
Irritation of the respiratory tract, as well as bronchial asthma or a history of bronchial asthma, severe chronic obstructive pulmonary disease.
Sinus bradycardia, sick sinus syndrome, as well as sinoatrial block, II-III degree block, which is not controlled by a pacemaker, severe heart failure or cardiogenic shock.
Side Effects
Side Effects
In clinical studies, more than 484 patients were treated with Taptiq ®. A common treatment-related adverse reaction reported was conjunctival flushing/redness. It occurred in approximately 7% of patients participating in clinical trials; it was mild in most cases and was associated with treatment discontinuation in 1.2% of patients.
Adverse reactions reported in clinical studies using Tapticom ® were limited to those adverse reactions previously reported with one of the active ingredients, tafluprost or timolol. No new adverse reactions characteristic of Tapticom ® were observed in clinical studies. Most adverse reactions reported were ocular, mild or moderate in severity, and were not serious.
Like other topical ophthalmic drugs, tafluprost and timolol are absorbed systemically. This may cause similar adverse reactions to those seen with systemic beta blockers. The incidence of systemic adverse reactions after topical use of ophthalmic drugs is lower than with systemic use. The following adverse reactions include those reactions that have been observed within the ophthalmic beta blocker class.
These adverse reactions were reported when taking the drug Tapticom ® in clinical studies (within each frequency group below, adverse reactions are presented in decreasing order of frequency).
The frequency of possible adverse reactions listed below was determined using the following conventions: very often (≥1/10); often (≥ 1/100 to < 1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000) very rare (<1/10,000) unknown (frequency cannot be determined from existing data).
Tapticom ® (tafluprost/timolol combination)
From the nervous system
Uncommon: headache
From the organs of vision
Common: conjunctiva/redness, itchy eyes, eye pain, changes in eyelashes (increase in length, thickness and number of eyelashes), discoloration of eyelashes, eye irritation, foreign body sensation in the eyes, blurred vision, photophobia (photophobia).
Uncommon: unusual sensation in the eyes, dry eyes, eye discomfort, conjunctivitis, erythema of the eyelids, eye allergies, swelling of the eyelids, superficial punctate keratitis, increased lacrimation, inflammation of the anterior chamber of the eye, asthenopia (easy eye fatigue), blepharitis (inflammation of the eyelids).
Additional adverse reactions that were observed when using one of the active ingredients (tafluprost or timolol) and may also occur when using the drug Tapticom ® are listed below.
Tafluprost
From the organs of vision: Reduced visual acuity, increased pigmentation of the iris, pigmentation of the eyelids, swelling of the conjunctiva, discharge from the eyes, cellular reaction of the moisture of the anterior chamber, cellular opalescence in the anterior chamber of the eye, allergic conjunctivitis, pigmentation of the conjunctiva, conjunctival follicles, deepening of the eyelid sulcus, iritis (inflammation of the iris) / uveitis (inflammation of the choroid of the eyeball).
From the skin and its derivatives: hypertrichosis of the eyelids.
From the respiratory system: exacerbation of bronchial asthma, shortness of breath (shortness of breath/difficulty breathing).
timolol
From the immune system: signs and symptoms of allergic reactions, including angioedema, urticaria, single and multiple rashes, anaphylactic reaction, itching.
Metabolism and nutrition: hypoglycemia.
From the mental side: depression, sleep disturbance (insomnia), nightmares, memory loss, nervousness.
From the nervous system: dizziness, fainting, paresthesia, increased myasthenia gravis, hemorrhagic stroke, cerebral ischemia.
From the organs of vision: keratitis, reduced sensitivity of the cornea, visual disturbances and disorders, including changes in refraction (due to the withdrawal of miotic drugs in some cases), ptosis (drooping of the eyelid), diplopia (double vision), choroidal detachment after filtering surgery, lacrimation, corneal erosion.
Hearing and balance disorders: ringing/tinnitus.
Cardiac: bradycardia, chest pain, palpitations, edema, arrhythmia, congestive heart failure, cardiac arrest, heart block, heart block, heart failure.
Vascular disorders: hypotension, claudication, Raynaud’s disease, cold extremities (hands and feet).
Respiratory, thoracic and mediastinal disorders: dyspnea (shortness of breath/difficulty breathing), bronchospasm (especially in patients with pre-existing bronchospastic disease), respiratory distress, cough.
From the digestive system: nausea, dyspepsia (indigestion), diarrhea, dry mouth, dysgeusia (taste disorder), abdominal pain, vomiting.
From the skin and its derivatives: alopecia (baldness), psoriasis-like rashes or exacerbation of psoriasis, skin rash.
From the musculoskeletal and connective tissue side: systemic lupus erythematosus, myalgia (muscle pain), arthropathy (joint disease).
From the genital organs and mammary glands: Peyronie’s disease (fibroplastic induration of the penis), decreased sexual desire (libido), sexual dysfunction.
General disorders and reactions at the injection site: asthenia (general weakness)/fatigue, thirst.
Cases of corneal calcification have been reported very rarely in association with the use of phosphate-containing eye drops in some patients with significant corneal damage.
Interaction
Interaction
No specific interaction studies with other drugs have been conducted.
Possible additive effects resulting in hypotension and/or severe bradycardia when beta blocker ophthalmic solution is used concomitantly with oral calcium channel blockers, beta blockers, antiarrhythmics (including amiodarone), digitalis glycosides, parasympathomimetics, guanethidine.
Oral beta-blockers may exacerbate rebound hypertension that occurs after discontinuation of clonidine.
Increased effects of systemic beta-blockers (e.g., decreased heart rate, depression) have been reported during combination treatment with CYP2D6 inhibitors (such as quinidine, fluoxetine, paroxetine) and timolol.
Mydriasis caused by concomitant use of ophthalmic beta blockers and epinephrine has been rarely reported.
Overdose
Overdose
Overdose with topical tafluprost is unlikely to occur or be associated with toxicity.
There have been reports of accidental overdose with timolol, resulting in symptoms of general poisoning similar to those observed with the use of systemic beta-blockers, such as dizziness, headache, difficulty breathing (dyspnea), bradycardia, bronchospasm and cardiac arrest (see also section “Adverse reactions”).
If an overdose of Taptiq ® occurs, treatment should be symptomatic and supportive. Timolol is not actively removed by hemodialysis.
Manufacturer
Manufacturer
Laboratory Unitere, France
Additional information
| Manufacturer | Laboratoire Uniter, France |
|---|---|
| Medication form | eye drops |
| Brand | Laboratoire Uniter |
Related products
Buy Tapticom, eye drops 0.0015%+0.5% 0.3 ml tube-caps 30 pcs with delivery to USA, UK, Europe and over 120 other countries.