No products in the cart.
Tabex, 1.5 mg 100 pcs
€52.25 €44.87
Description
Clinico-pharmacological group
H-cholinomimetic.
Pharmacological action
Activates nicotinic receptors of autonomic ganglia, reflexively stimulates respiratory center, causes release of adrenaline by chromaffin cells of medullary part of adrenal glands, increases BP.
Limits nicotine dependence (due to the competitive relationship in the area of the same receptors and biochemical substrates with which nicotine interacts in the body).
Causes a change in the taste of smoking (making it unpleasant), reduces the urge to smoke and eases the withdrawal symptoms associated with stopping smoking.
Indications
Indications
Nicotine addiction (to facilitate smoking cessation).
Physical and psychological dependence on nicotine is considered a specific type of disease that results in the inability to abstain from smoking, even with the understanding of its negative effects.
Pharmacological effect
Pharmacological effect
Pharmacological action
n-cholinomimetic.
Pharmacodynamics
The alkaloid cytisine, which is the active substance of the drug, has an n-cholinomimetic effect; excites the ganglia of the autonomic nervous system, reflexively stimulates the respiratory center, causes the release of adrenaline from the adrenal medulla, increases blood pressure.
While closely similar to the mechanism of action of nicotine, cytisine has much lower toxicity and a greater therapeutic index.
Cytisine competitively inhibits the interaction of nicotine with the corresponding receptors, which leads to a gradual decrease and disappearance of nicotine addiction.
Pharmacokinetics
No pharmacokinetic studies have been conducted in humans.
Special instructions
Special instructions
The drug should be started only when the patient has a serious intention to quit smoking.
Persons with a long history of smoking and persons over 40–45 years of age should use Tabex® only after consultation with a doctor. When using the drug, do not exceed the recommended dose. Treatment with the drug and continued smoking may lead to increased side effects of nicotine (nicotine intoxication). The medicinal product contains the excipient lactose. Patients with rare hereditary problems of galactose intolerance, lactase deficiency (Lapp type) or glucose-galactose malabsorption should not use the drug.
Impact on the ability to drive vehicles and operate machinery. Although no negative effects on the ability to drive vehicles or operate machinery were observed during the use of the drug, due to the possible occurrence of drowsiness and other adverse reactions, caution should be exercised in the listed situations.
Conditions for dispensing from pharmacies
Without a prescription.
Active ingredient
Active ingredient
Cytisine
Composition
Composition
1 film-coated tablet contains:
Active ingredient:
cytisine 1.5 mg.
Excipients:
lactose monohydrate – 28 mg; MCC – 67.5 mg; talc – 2 mg; magnesium stearate – 1 mg
Film shell composition:
Opadry II brown 8 F 26948 – 3 mg: partially hydrolyzed polyvinyl alcohol – 1.2 mg; titanium dioxide (E171) – 0.654 mg; macrogol 3350 – 0.606 mg; talc – 0.444 mg; iron oxide yellow (E172) – 0.062 mg, iron oxide red (E172) – 0.018 mg, iron oxide black (E172) – 0.016 mg
Pregnancy
Pregnancy
Tabex® should not be used by women during pregnancy and breastfeeding.
Contraindications
Contraindications
hypersensitivity to the active or any of the excipients of the drug;
acute myocardial infarction;
unstable angina;
cardiac arrhythmia;
recent cerebrovascular accident;
atherosclerosis;
severe arterial hypertension;
lactase deficiency, galactosemia, glucose-galactose malabsorption syndrome (the drug contains lactose);
pregnancy;
breastfeeding period;
age under 18 and after 65 years.
With caution: other forms of coronary heart disease (stable angina, asymptomatic (silent) myocardial ischemia, vasospastic angina, syndrome “X” (microvascular angina); heart failure, high blood pressure; cerebrovascular disease; obliterating arterial diseases, hyperthyroidism, gastric ulcer, diabetes mellitus, renal or hepatic failure, some forms schizophrenia, the presence of chromaffin tumors of the adrenal glands; gastroesophageal reflux disease; persons with a long history of smoking and persons over 40–45 years of age (see “Special instructions”).
Side Effects
Side Effects
The drug is usually well tolerated, the observed side effects are mild or moderate. Most of them appear at the beginning of treatment and go away on their own. Most often they are associated with quitting smoking and are manifested by dizziness, headache and insomnia.
At recommended doses, Tabex® does not cause serious adverse effects.
The following side effects are possible:
From the cardiovascular system: tachycardia, slight increase in blood pressure, palpitations.
From the central nervous system: headache, dizziness, insomnia or drowsiness, increased irritability.
From the respiratory system: shortness of breath.
From the gastrointestinal tract: dry mouth, nausea, abdominal pain, constipation, diarrhea, changes in taste and appetite.
Musculoskeletal and connective tissue disorders: muscle pain.
Metabolism and nutrition: weight loss, increased sweating.
Other: chest pain.
Interaction
Interaction
After smoking cessation, the side effects of theophylline, ropinirole, clozapine and olanzapine may increase.
With the simultaneous use of the drug Tabex® with acetylcholine, carbachol, galantamine, pyridostigmine, rivastigmine, distigmine, it is possible to increase cholinomimetic side effects (salivation, lacrimation, bronchial secretion with cough and the risk of an asthma attack, constriction of the pupil, colic, nausea, vomiting, frequent urination, increased muscle tone or sudden muscle contractions).
The use of Tabex® simultaneously with lovastatin, simvastatin, fluvastatin, pravastatin, etc. increases the risk of muscle pain.
The simultaneous use of Tabex® with antihypertensive drugs (propranolol, etc.) may weaken their effect.
Overdose
Overdose
Symptoms: nausea, vomiting, dilated pupils, general weakness, tachycardia, clonic convulsions, respiratory paralysis. Occurs when the patient does not comply with the dosage regimen and takes the drug in doses several times higher than therapeutic.
Treatment: gastric lavage, administration of activated charcoal, infusion of water-salt solutions, as well as 5 or 10% glucose solution, administration of anticonvulsants, cardiotonics, respiratory analeptics and other symptomatic agents. Respiratory function, blood pressure and heart rate should be monitored.
Storage conditions
Storage conditions
Store in original packaging to protect from light and moisture, at a temperature not exceeding 25 °C.
Keep out of the reach of children.
Shelf life
Shelf life
2 years.
Do not use after the expiration date indicated on the package.
Manufacturer
Manufacturer
Sopharma JSC, Bulgaria
Additional information
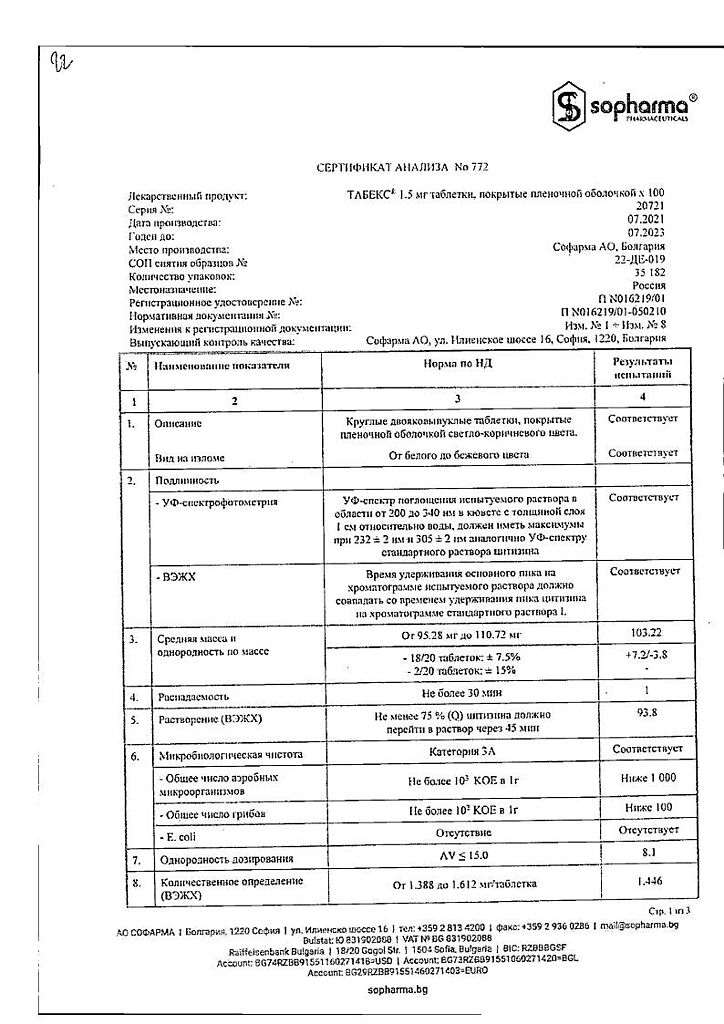
| Shelf life | 2 years. |
|---|---|
| Conditions of storage | The drug Tabex should be stored in a dry place, protected from light, out of the reach of children at a temperature not exceeding 25°C. |
| Manufacturer | Sofarma JSC, Bulgaria |
| Medication form | pills |
| Brand | Sofarma JSC |
Related products
Buy Tabex, 1.5 mg 100 pcs with delivery to USA, UK, Europe and over 120 other countries.