No products in the cart.
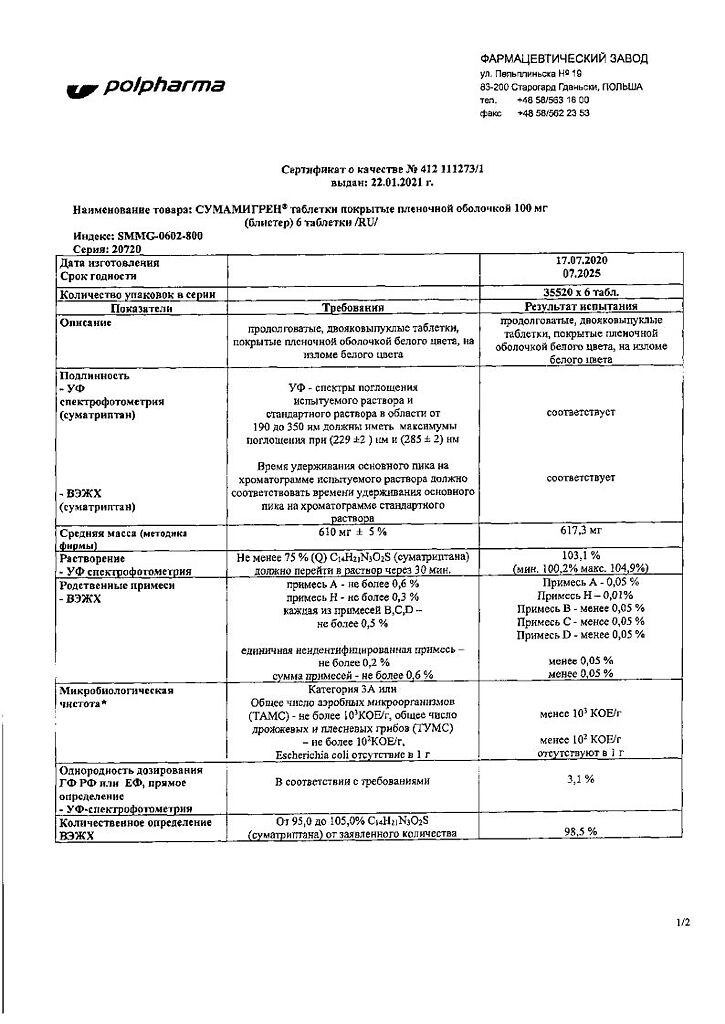
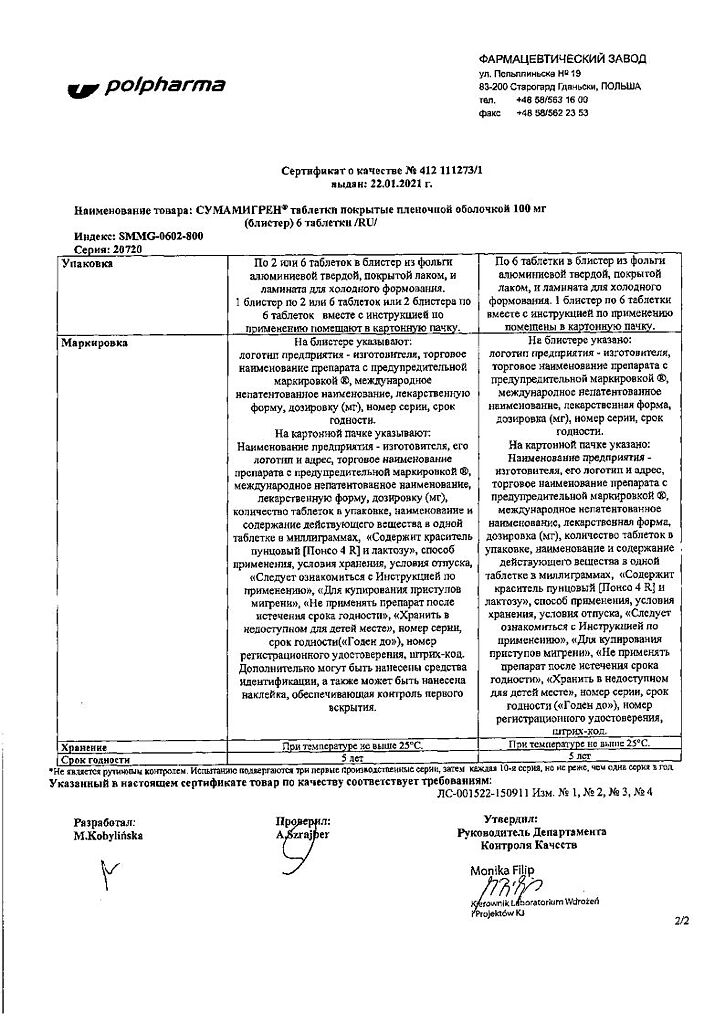
Sumamigren, 100 mg 6 pcs
€25.33 €21.11
Description
The pharmacological action is serotoninergic, antimigraine.
Sumatriptan is a specific selective agonist of vascular 5-hydroxytryptamine-1 receptors (5HT1D), does not affect other 5HT-serotonin receptor subtypes (5HT2-5HT7). The 5HT1D receptors are located mainly in the blood vessels of the brain, and their stimulation results in the narrowing of these vessels. Decreases the sensitivity of the trigeminal nerve. Both of these effects may underlie the antimigraine action of sumatriptan. Clinical effect is usually noted 30 min after oral administration.
Pharmacokinetics
Sumatriptan is rapidly absorbed after oral administration, 70% of the Cmax is reached in 45 min. After administration of 100 mg, the Cmax in plasma averages 54 ng/mL. Bioavailability is 14% due to intensive presystemic metabolism and incomplete absorption. Binding to plasma proteins is low (14-21%).
Sumatriptan is metabolized by MAO A. The main metabolite, the indole acetic analog of sumatriptan, is excreted primarily in the urine, as free acid and glucuronide conjugate. This metabolite has no activity toward 5NT1– and 5NT2-serotonin receptors. Migraine attacks do not appear to have a significant effect on the pharmacokinetics of sumatriptan taken orally.
Indications
Indications
Relief of migraine attacks with or without aura.
Pharmacological effect
Pharmacological effect
Pharmacological action – serotonergic, antimigraine.
Sumatriptan is a specific selective agonist of vascular 5-hydroxytryptamine-1 receptors (5HT1D), does not affect other subtypes of 5HT-serotonin receptors (5HT2–5HT7). 5HT1D receptors are located primarily in the blood vessels of the brain, and their stimulation causes these vessels to constrict. Reduces the sensitivity of the trigeminal nerve. Both of these effects may underlie the antimigraine action of sumatriptan. The clinical effect is usually observed 30 minutes after taking the drug orally.
Pharmacokinetics
After oral administration, sumatriptan is rapidly absorbed, 70% of Cmax is achieved after 45 minutes. After taking 100 mg, Cmax in blood plasma averages 54 ng/ml. Bioavailability is 14% due to intensive first-pass metabolism and incomplete absorption. Plasma protein binding is low (14–21%).
Sumatriptan is metabolized by the action of MAO A. The main metabolite, the indoleacetic analogue of sumatriptan, is excreted primarily in the urine, in the form of a free acid and a glucuronide conjugate. This metabolite has no activity at 5HT1- and 5HT2-serotonin receptors. Migraine attacks do not appear to have a significant effect on the pharmacokinetics of oral sumatriptan.
Special instructions
Special instructions
Sumatriptan should only be prescribed if the diagnosis of migraine is certain and should be used as soon as possible after the onset of a migraine attack, although it is equally effective when used at any stage of the attack.
The drug cannot be used for prophylactic purposes.
Sumatriptan should be used with caution in patients with controlled hypertension; diseases in which the absorption, metabolism or excretion of the drug may be altered (for example, impaired renal or liver function).
There are very rare reports from post-marketing surveillance of the development of serotonin syndrome (including mental disorders, autonomic lability and neuromuscular disorders) as a result of concomitant use of selective serotonin reuptake inhibitors (SSRIs) and sumatriptan. The development of serotonin syndrome has also been reported during concomitant use of triptans with selective serotonin norepinephrine reuptake inhibitors (SNRIs). In case of simultaneous administration with drugs from the SSRI/SNRI group, the patient’s condition should be carefully monitored.
Sumatriptan should be used with caution in epilepsy and any conditions with a decreased seizure threshold.
Concomitant use of other triptans/5-HT1 agonists with sumatriptan is not recommended.
In patients with hypersensitivity to sulfonamides, the use of sumatriptan may cause allergic reactions, the severity of which varies from skin manifestations to anaphylaxis. Cross-sensitivity data are limited, but caution should be exercised when prescribing sumatriptan to such patients.
As with other antimigraine drugs, when prescribing sumatriptan in patients with previously undiagnosed migraine or in patients with atypical migraine, it is necessary to exclude other potentially serious neurological conditions. It should be noted that patients with migraine have an increased risk of developing certain cerebrovascular complications (stroke or transient cerebrovascular accident).
Sumatriptan should not be prescribed to patients with suspected heart disease without prior evaluation to exclude cardiovascular pathology. These patients include postmenopausal women, men over 40 years of age, and patients with risk factors for coronary artery disease. Although testing may not always detect heart disease in some patients, in very rare cases they develop cardiovascular side effects. After taking sumatriptan, transient intense pain and tightness in the chest may occur, spreading to the neck area. If there is reason to believe that these symptoms are a manifestation of coronary artery disease, it is necessary to conduct an appropriate diagnostic examination.
Overuse of medications intended to treat migraine attacks is associated with increased headaches in sensitive patients (drug overuse headache). In this case, the possibility of discontinuing the drug should be considered.
Do not exceed the recommended dose of sumatriptan.
Impact on the ability to drive vehicles and operate machinery
Patients with migraine may experience drowsiness associated both with the disease itself and with taking sumatriptan, so they should be especially careful when driving or operating moving machinery.
Active ingredient
Active ingredient
Sumatriptan
Composition
Composition
Film-coated tablets
1 table
sumatriptan succinate
140 mg
(corresponds to 100 mg sumatriptan)
excipients:
lactose;
MCC;
croscarmellose sodium;
magnesium stearate;
talc;
silicon dioxide colloidal anhydrous
shell:
hypromellose;
macrogol 6000;
talc;
titanium dioxide;
triethyl citrate;
orange-yellow varnish E110
Pregnancy
Pregnancy
The use of sumatriptan is contraindicated during pregnancy. Breastfeeding should be stopped during treatment. If you take the drug, breastfeeding is possible no earlier than 24 hours later.
Use in children
Contraindication: patients under 18 years of age.
Contraindications
Contraindications
individual increased sensitivity to any component of Sumamigren;
hemiplegic, basilar and ophthalmoplegic forms of migraine;
IHD (including myocardial infarction, post-infarction cardiosclerosis, Prinzmetal’s angina), as well as the presence of symptoms suggesting the presence of IHD;
occlusive diseases of peripheral vessels;
stroke or transient ischemic attack (including history);
uncontrolled arterial hypertension;
simultaneous use with ergotamine or its derivatives (including metisegride);
use while taking MAO inhibitors or earlier than 2 weeks after their discontinuation;
severe dysfunction of the liver and/or kidneys;
age under 18 years and over 65 years (safety and effectiveness have not been established);
pregnancy and lactation period.
Side Effects
Side Effects
From the side of the body as a whole
Pain, heat, tingling, tightness or heaviness (usually transient, but can be intense and occur in various parts of the body, including the chest or throat); Hot flashes, dizziness, a feeling of weakness, a feeling of fatigue, drowsiness are also possible (usually mild or moderate, transient).
From the cardiovascular system
Decreased blood pressure, bradycardia, tachycardia, transient increase in blood pressure; rarely – rhythm disturbances, transient ischemic ECG changes, coronary artery spasm, myocardial infarction; in isolated cases – Raynaud’s syndrome.
From the digestive system
Nausea, vomiting, ischemic colitis (the connection between these phenomena and the use of sumatriptan has not been clearly established); feeling of discomfort in the abdomen, dysphagia, increased activity of liver transaminases.
From the side of the central nervous system
Dizziness; rarely – seizures (in some cases observed in patients with a history of seizures or with conditions predisposing to the development of seizures); sometimes – diplopia, scotoma, nystagmus, decreased visual acuity; extremely rarely – partial transient loss of vision (it should be borne in mind that visual impairment may be associated with a migraine attack).
Allergic reactions
Rash, itching, erythema, urticaria; in isolated cases – anaphylactic reactions.
Interaction
Interaction
There were no drug interactions between sumatriptan and propranolol, flunarizine, pizotifen and ethanol.
Prolonged vasospasm was observed when taken concomitantly with ergotamine. Sumatriptan can be prescribed no earlier than 24 hours after taking drugs containing ergotamine, and drugs containing ergotamine can be prescribed no earlier than 6 hours after taking sumatriptan.
The simultaneous use of sumatriptan and a MAO inhibitor is contraindicated, because interaction between them is possible.
There are very rare reports from post-marketing surveillance of the development of serotonin syndrome (including mental disorders, autonomic lability and neuromuscular disorders) as a result of concomitant use of SSRIs and sumatriptan. The development of serotonin syndrome has also been reported during concomitant use of triptans with SNRIs.
Overdose
Overdose
In case of overdose, the patient should be observed for 10 hours, providing symptomatic therapy as necessary.
There are no data on the effect of hemodialysis or peritoneal dialysis on plasma concentrations of sumatriptan.
Storage conditions
Storage conditions
The drug should be stored out of the reach of children at a temperature not exceeding 25°C.
Shelf life
Shelf life
5 years.
Manufacturer
Manufacturer
Polpharma JSC, Poland
Additional information
| Shelf life | 5 years. |
|---|---|
| Conditions of storage | The drug should be kept out of reach of children at a temperature not exceeding 25 ° C. |
| Manufacturer | Polpharma S.A., Poland |
| Medication form | pills |
| Brand | Polpharma S.A. |
Other forms…
Related products
Buy Sumamigren, 100 mg 6 pcs with delivery to USA, UK, Europe and over 120 other countries.