No products in the cart.
Stopdiar, 220 mg/5 ml suspension 90 ml
€15.26 €12.72
Description
Pharmacodynamics
Nifuroxazide – intestinal antiseptic, 5-nitrofuran derivative; active against most pathogens of intestinal infections (including mutant strains resistant to other antimicrobials): Gram-positive (family Staphylococcus) and Gram-negative (family Enterobacteriaceae: Escherichia, Citrobacter, Enterobacter, Klebsiella, Salmonella, Shigella, Proteus, Yersinia), and Vibrio cholerae. It is not active against bacteria of the genus Pseudomonas and the genus Proteus (species Proteus inconstans), as well as subgroup A strains of Providentia alcalifaciens. It is assumed that the drug inhibits dehydrogenase activity and disrupts protein synthesis in pathogenic bacteria. In medium therapeutic doses it has bacteriostatic activity, and in higher doses it has bactericidal activity. The effect is seen from the first hours of treatment.
In therapeutic doses it practically does not disturb the balance of the symbiotic bacterial flora of the intestine; does not cause development of the resistant strains of pathogenic microorganisms and cross-resistance of bacteria to other antimicrobial agents which allows to prescribe it in complex therapy with systemic agents if necessary for generalized infections. In intestinal infections of viral genesis it prevents the development of bacterial superinfection.
Pharmacokinetics
After oral administration it is practically not absorbed from the digestive tract, creating a high concentration of the active substance in the intestine. Due to these pharmacokinetic peculiarities the drug produces exclusively enteric antiseptic action, it has no systemic antibacterial activity and no general toxic effects; it is excreted with feces. The drug has no effect on clinical and biochemical parameters of blood analysis.
Indications
Indications
Acute and chronic diarrhea caused by intestinal gram-positive bacteria (Staphylococcus spp., Streptococcus spp., Haemophylus influenzae), as well as some gram-negative bacteria (Salmonella, Shigella, Klebsiella, Escherichia coli, Proteus spp., Enterobacter spp.).
Pharmacological effect
Pharmacological effect
Pharmacodynamics
Nifuroxazide is an intestinal antiseptic, a derivative of 5-nitrofuran; active against most pathogens of intestinal infections (including mutant strains resistant to other antimicrobial agents): gram-positive (Staphylococcus family) and gram-negative (Enterobacteriaceae family: Escherichia, Citrobacter, Enterobacter, Klebsiella, Salmonella, Shigella, Proteus, Yersinia), as well as Vibrio cholerae. It is not active against bacteria of the genus Pseudomonas and the genus Proteus (species Proteus inconstans), as well as strains of subgroup A of the species Providentia alcalifaciens. It is assumed that the drug inhibits the activity of dehydrogenases and disrupts protein synthesis in pathogenic bacteria. At medium therapeutic doses it has bacteriostatic activity, and at higher doses it has a bactericidal effect. The effect appears from the first hours of treatment.
In therapeutic doses, it practically does not disturb the balance of the symbiotic bacterial flora of the intestine; does not cause the development of resistant strains of pathogenic microorganisms and cross-resistance of bacteria to other antimicrobial agents, which allows, if necessary, for generalized infections to prescribe it in combination therapy with systemic drugs. In case of intestinal infections of viral origin, it prevents the development of bacterial superinfection.
Pharmacokinetics
After oral administration, it is practically not absorbed from the digestive tract, creating a high concentration of the active substance in the intestine. Thanks to these pharmacokinetic properties, the drug has an exclusively enteral antiseptic effect, does not have systemic antibacterial activity, and does not cause general toxic effects; excreted from the body with feces. The drug does not affect the clinical and biochemical parameters of blood tests.
Special instructions
Special instructions
When treating diarrhea, rehydration therapy must be carried out simultaneously with nifuroxazide therapy.
Treatment of diarrhea in children under 3 years of age is recommended under medical supervision.
Active ingredient
Active ingredient
Nifuroxazide
Composition
Composition
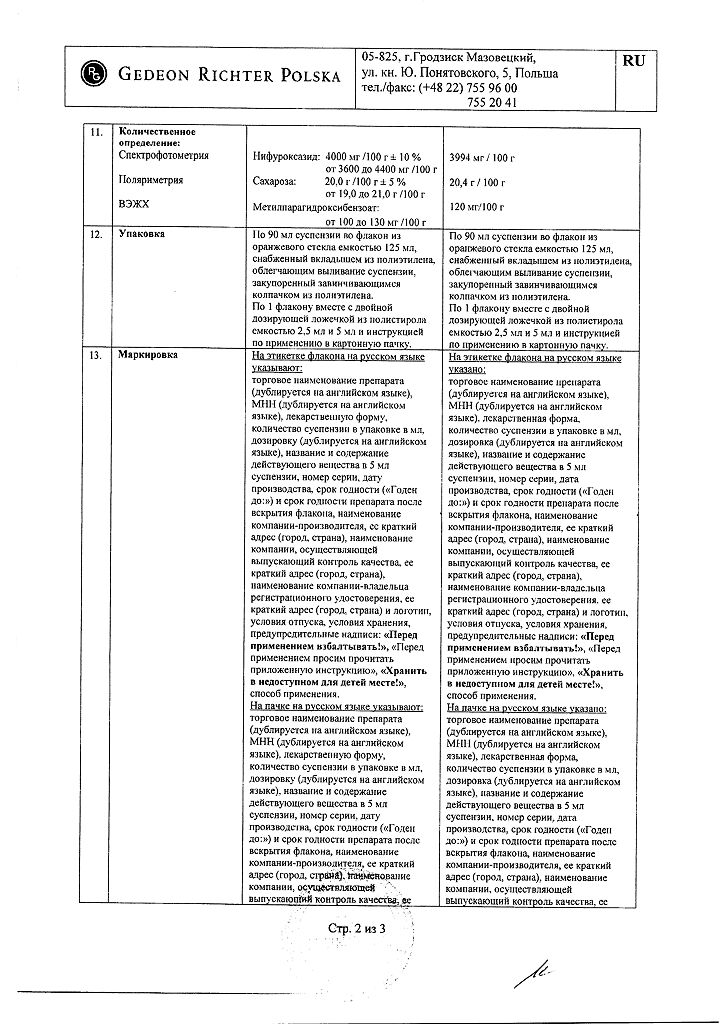
5 ml of suspension contains:
Active substances:
nifuroxazide 220 mg.
Excipients:
carbomer – 11 mg,
sucrose – 1100 mg,
sodium hydroxide – 2.2 mg,
citric acid monohydrate – 0.83 mg,
simethicone emulsion 30% (anti-foam emulsion) – 11 mg,
methyl parahydroxybenzoate – 5.5 mg,
banana flavor – 4.57 mg,
water – up to 5 ml.
Pregnancy
Pregnancy
There is no data regarding adverse effects on the fetus when using the drug during pregnancy.
If necessary, with caution, the drug can be prescribed to pregnant and breastfeeding women.
Contraindications
Contraindications
Hypersensitivity to nifuroxazide, nitrofuran derivatives or any excipients of the drug;
Side Effects
Side Effects
Allergic reactions (skin rash, urticaria, Quincke’s edema, anaphylactic shock).
Interaction
Interaction
Concomitant use with drugs that cause the development of disulfiram-like reactions or drugs that inhibit the function of the central nervous system is not recommended.
Overdose
Overdose
Overdose symptoms are not described.
In case of overdose, gastric lavage and symptomatic treatment are recommended.
Storage conditions
Storage conditions
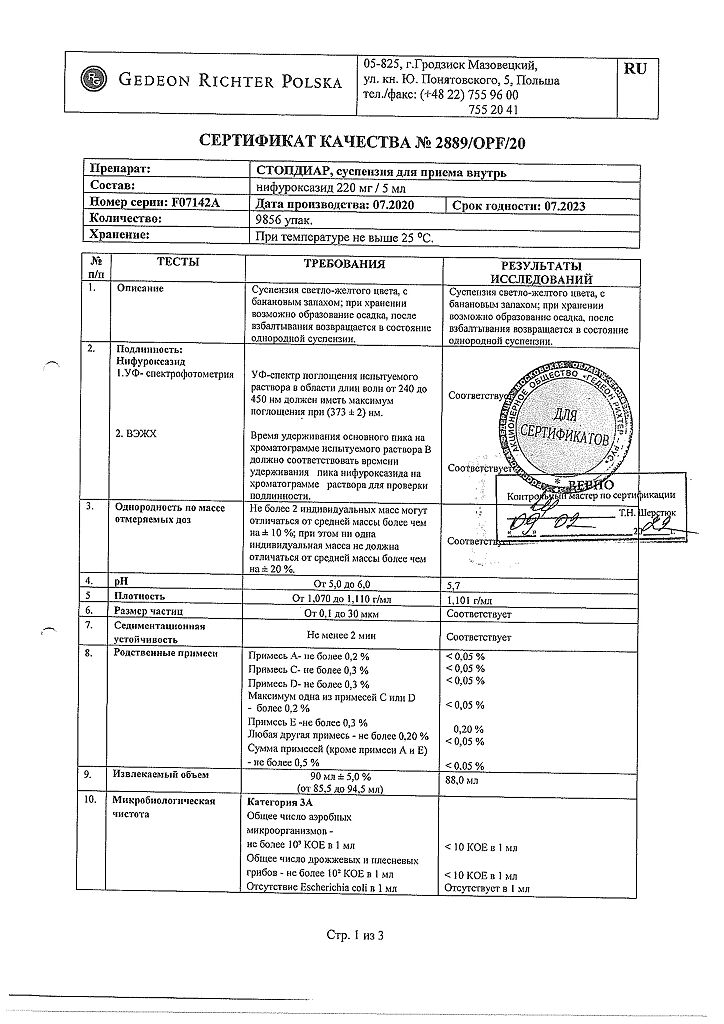
At a temperature not exceeding 25 °C
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Gedeon Richter, Hungary
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | At a temperature not exceeding 25 °C |
| Manufacturer | Gedeon Richter, Hungary |
| Medication form | oral suspension |
| Brand | Gedeon Richter |
Other forms…
Related products
Buy Stopdiar, 220 mg/5 ml suspension 90 ml with delivery to USA, UK, Europe and over 120 other countries.