No products in the cart.
Sirdalud MR, 6 mg 30 pcs
€15.09 €12.57
Out of stock
(E-mail when Stock is available)
Description
Pharmgroup:
Myorelaxant of central action.
Pharmic action:
Tizanidine is a centrally acting muscle relaxant. Its main point of action is in the spinal cord. By stimulating presynaptic α2-receptors in the spinal cord, tizanidine suppresses release of excitatory amino acids which stimulate NMDA-receptors. As a consequence, polysynaptic excitation transmission is inhibited at the level of intermediate spinal cord neurons. Since it is this mechanism that is responsible for excess muscle tone, when it is suppressed, muscle tone is reduced. In addition to its myorelaxant properties, tizanidine also has a moderately pronounced central analgesic effect.
Sirdalud® is effective for both acute painful muscle spasm and chronic spasticity of spinal and cerebral genesis. It reduces spasticity and clonic spasms, as a result of which resistance to passive movements decreases and the volume of active movements increases.
The myorelaxant effect (as measured with the Ashworth scale and pendulum test) and adverse effects (decrease in HR and BP) of Sirdalud® depend on the plasma concentration of the drug.
Pharmacokinetics:
Intake
Tizanidine is absorbed rapidly and almost completely. Cmax in plasma is reached approximately 1 h after taking the drug. Because of the pronounced metabolism during “first passage” through the liver, the average bioavailability is about 34%.
The cmax of tizanidine is 12.3 ng/ml and 15.6 ng/ml after single and multiple doses of tizanidine at a dose of 4 mg, respectively.
Simultaneous intake of food does not affect pharmacokinetics of tizanidine. Although the Cmax value increases by 1/3 when the tablet is taken after a meal, this is not considered to be clinically significant. There is no significant effect on absorption (AUC). Tizanidine in the dose range from 1 mg to 20 mg has linear pharmacokinetics.
Distribution
The binding to plasma proteins is 30%.
Metabolism
Tizanidine is rapidly and largely (about 95%) metabolized in the liver. In vitro it has been shown that tizanidine is mainly metabolized by cytochrome P450 system isoenzyme 1A2. The metabolites are inactive.
The average T1/2 of tizanidine from the systemic bloodstream is 2-4 hours. The drug is excreted primarily by the kidneys (approximately 70% of the dose) as metabolites; a share of unchanged substance is only about 4.5%.
Pharmacokinetics in special clinical cases
. In patients with renal insufficiency (creatinine clearance ≤ 25 ml/min) the average Cmax of the drug in plasma is 2 times higher than in healthy volunteers, and the final T1/2 reaches 14 h, which leads to increased (approximately 6-fold) systemic bioavailability of tizanidine (measured by AUC)
Pharmacokinetics of tizanidine/p>
There have been no specific studies in patients with impaired liver function.
Tizanidine is primarily metabolized in the liver, by cytochrome CYP1A2; therefore, impairment of liver function may increase systemic exposure to the drug.
There are limited data on pharmacokinetics in patients over 65 years of age.
Gender has no effect on the pharmacokinetic properties of tizanidine.
The effect of ethnicity and race on the pharmacokinetics of tizanidine has not been studied.
Indications
Indications
Painful muscle spasms in diseases of the spine (including osteochondrosis, spondylosis, syringomyelia, hemiplegia, cervical and lumbar syndromes);
After surgery for a herniated disc or osteoarthritis of the hip,
Spastic state of skeletal muscles caused by neurological diseases: multiple sclerosis, chronic myelopathy, degenerative diseases of the spinal cord, cerebrovascular accidents, stroke, traumatic brain injury, cerebral palsy, seizures of central origin.
Pharmacological effect
Pharmacological effect
Pharmaceutical group:
centrally acting muscle relaxant.
Pharmaceutical action:
Tizanidine is a centrally acting muscle relaxant. The main point of application of its action is in the spinal cord. By stimulating presynaptic α2 receptors in the spinal cord, tizanidine inhibits the release of excitatory amino acids that stimulate NMDA receptors. As a result, polysynaptic transmission of excitation is suppressed at the level of interneurons of the spinal cord. Since it is this mechanism that is responsible for excess muscle tone, when it is suppressed, muscle tone decreases. In addition to its muscle relaxant properties, tizanidine also has a moderate central analgesic effect.
Sirdalud® is effective both in acute painful muscle spasms and in chronic spasticity of spinal and cerebral origin. Reduces spasticity and clonic convulsions, as a result of which resistance to passive movements decreases and the range of active movements increases.
The muscle relaxant effect (measured on the Ashworth scale and using the “pendulum” test) and side effects (decrease in heart rate and blood pressure) of Sirdalud® depend on the concentration of the drug in the blood plasma.
Pharmacokinetics:
Suction
Tizanidine is absorbed quickly and almost completely. Cmax in plasma is achieved approximately 1 hour after taking the drug. Due to the pronounced first-pass metabolism through the liver, the average bioavailability is about 34%.
Cmax of tizanidine is 12.3 ng/ml and 15.6 ng/ml after single and multiple doses of tizanidine 4 mg, respectively.
Concomitant food intake does not affect the pharmacokinetics of tizanidine. Although the Cmax value increases by 1/3 when the tablet is taken after a meal, this is not considered to be clinically significant. There is no significant effect on absorption (AUC). Tizanidine in the dose range from 1 mg to 20 mg has linear pharmacokinetics.
Distribution
Plasma protein binding is 30%.
Metabolism
Tizanidine is rapidly and extensively (about 95%) metabolized in the liver. In vitro, tizanidine has been shown to be primarily metabolized by cytochrome P450 isoenzyme 1A2. Metabolites are inactive.
Removal
The average half-life of tizanidine from the systemic circulation is 2-4 hours. The drug is excreted primarily by the kidneys (approximately 70% of the dose) in the form of metabolites; the share of unchanged substance accounts for only about 4.5%.
Pharmacokinetics in special clinical situations
In patients with renal failure (creatinine clearance ≤ 25 ml/min), the average Cmax of the drug in plasma is 2 times higher than in healthy volunteers, and the final half-life reaches 14 hours, which leads to increased (about 6 times) systemic bioavailability of tizanidine (measured by AUC)
No specific studies have been conducted in patients with impaired liver function.
Tizanidine is predominantly metabolized in the liver by cytochrome CYP1A2, therefore, impaired liver function may lead to increased systemic exposure of the drug.
Pharmacokinetic data in patients over 65 years of age are limited.
Gender does not affect the pharmacokinetic properties of tizanidine.
The influence of ethnicity and race on the pharmacokinetics of tizanidine has not been studied.
Special instructions
Special instructions
Cases of liver dysfunction associated with tizanidine have been reported, but these cases are rare at daily doses up to 12 mg. In this regard, it is recommended to monitor liver function tests once a month in the first 4 months of treatment in those patients who are prescribed tizanidine at a daily dose of 12 mg or higher, as well as in cases where there are clinical signs suggesting impaired liver function, such as unexplained nausea, anorexia, and fatigue. In cases where serum ALT and AST levels persistently exceed the upper limit of normal by 3 times or more, the use of Sirdalud should be discontinued.
Since Sirdalud tablets contain lactose, it is not recommended to use the drug in patients with rare hereditary galactose intolerance, severe lactase deficiency or glucose/galactose malabsorption.
Impact on the ability to drive a car and operate machinery.
Patients who experience drowsiness or dizziness should refrain from activities that require high concentration and quick reactions, such as driving vehicles or working with machines and mechanisms.
Active ingredient
Active ingredient
Tizanidine
Composition
Composition
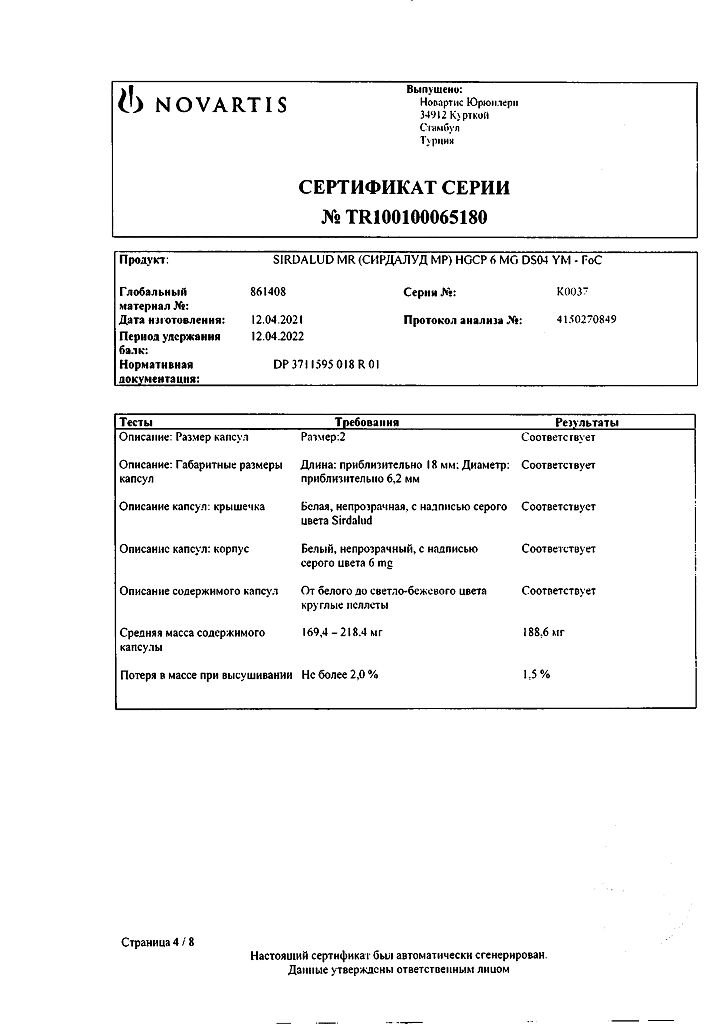
1 capsule contains:
active substance:
tizanidine (hydrochloride form) 6 mg
Pregnancy
Pregnancy
Since there are no controlled studies of tizanidine in pregnant women, it should not be used during pregnancy unless the potential benefit outweighs the potential risk.
Tizanidine is excreted in breast milk in small quantities; however, the drug should not be used by women breastfeeding children.
Contraindications
Contraindications
Hypersensitivity to tizanidine or any other component of the drug.
Severe liver dysfunction.
Concomitant use with fluvoxamine or ciprofloxacin.
With caution
Concomitant use of Sirdalud with CYP1A2 inhibitors is not recommended.
Side Effects
Side Effects
From the cardiovascular system: often – bradycardia, low blood pressure.
From the gastrointestinal tract: often – dry mouth; rarely – nausea, gastrointestinal disorders.
From the liver: rarely – increased activity of liver transaminases, very rarely – hepatitis.
From the musculoskeletal system: rarely – muscle weakness.
Other: often – fatigue.
When taking small doses recommended for relieving painful muscle spasms, drowsiness, fatigue, dizziness, dry mouth, decreased blood pressure (BP), nausea, gastrointestinal disorders, and increased activity of liver transaminases were observed. Typically, the above-described adverse reactions are moderate and transient.
When taking higher doses recommended for the treatment of spasticity, the above adverse reactions occur more often and are more severe, but they are rarely so severe that treatment has to be interrupted. In addition, the following phenomena may occur: decreased blood pressure, bradycardia, muscle weakness, insomnia, sleep disturbances, hallucinations, hepatitis.
Interaction
Interaction
The simultaneous use of tizanidine with fluvoxamine or ciprofloxacin, which are inhibitors of cytochrome P450 1A2, is contraindicated. Concomitant use of tizanidine with fluvoxamine or ciprofloxacin resulted in a 33-fold or 10-fold increase in tizanidine AUC, respectively.
The result of combined use may be a clinically significant and prolonged decrease in blood pressure, leading to drowsiness, dizziness, and inhibited psychomotor reactions. The simultaneous administration of tizanidine with other CYP1A2 inhibitors – antiarrhythmic drugs (amiodarone, mexiletine, propafenone), cimetidine, fluoroquinolones (enoxacin, pefloxacin, norfloxacin), rofecoxib, oral contraceptives, ticlopidine is not recommended.
Concomitant administration of Sirdalud with antihypertensive drugs, including diuretics, can sometimes cause a decrease in blood pressure and bradycardia. Alcohol or sedatives may increase the sedative effect of Sirdalud.
Overdose
Overdose
To date, there have been several reports of Sirdalud overdose, including a case where the dose taken was 400 mg. In all cases, recovery was uneventful.
Symptoms: nausea, vomiting, decreased blood pressure, dizziness, drowsiness, miosis, anxiety, respiratory failure, coma.
Treatment. To remove the drug from the body, repeated administration of activated carbon is recommended. Forced diuresis may also speed up the elimination of Sirdalud. Subsequently, symptomatic treatment is carried out.
Storage conditions
Storage conditions
At a temperature not exceeding 25 °C
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Novartis Saglik Gida ve Tarim Yrunleri Sanayi, Türkiye
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | At a temperature not exceeding 25 °C |
| Manufacturer | Novartis Saglik Gida ve Tarim Yurunleri Sanayi, Turkey |
| Medication form | modified-release capsules |
| Brand | Novartis Saglik Gida ve Tarim Yurunleri Sanayi |
Related products
Buy Sirdalud MR, 6 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.