No products in the cart.
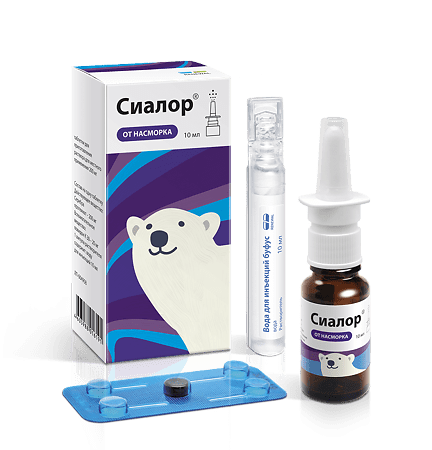
Sialor, set 2% 10 ml with sprayer
€12.85 €10.71
Description
Pharmacotherapeutic group: antiseptic.
ATX code: R01AX10
Pharmacological action
The drug of silver (silver proteinate contains 7.5-8.5% silver). It has astringent, antiseptic and anti-inflammatory effect. It dissociates with the formation of silver ions that bind with the DNA of bacteria and prevent their reproduction on mucous membranes in conditions of local application. Mechanism of action of silver proteinate is based on the fact that silver ions precipitate proteins and form a protective film on the damaged mucous membrane, which helps to reduce the sensitivity of nerve endings and narrowing of blood vessels (which leads to reduction of edema), which in turn leads to inhibition of inflammatory reactions. Silver ions also inhibit the reproduction of various bacteria. It is active against Gram-positive and Gram-negative microorganisms: B. cereus, C. albicans, E. coli, P. aeruginosa, S. aureus, A. niger, S. abony and others.
Pharmacokinetics
It is practically not absorbed when applied topically.
Indications
Indications
Symptomatic treatment of acute nasopharyngitis, sinusitis; acute rhinitis (runny nose).
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: drugs for the treatment of nasal diseases; decongestants and other drugs for topical use; other drugs for the treatment of nasal diseases.
ATX code: R01AX10
Pharmacological properties
Pharmacodynamics
Silver preparation (silver proteinate contains 7.5-8.5% silver). Has an astringent, antiseptic and anti-inflammatory effect. Dissociates to form silver ions, which bind to bacterial DNA and prevent their proliferation on mucous membranes when applied topically. The mechanism of action of silver proteinate is based on the fact that silver ions precipitate proteins and form a protective film on the damaged mucous membrane, which helps reduce the sensitivity of nerve endings and narrow blood vessels (this leads to a decrease in swelling), which in turn inhibits inflammatory reactions. Silver ions also inhibit the proliferation of various bacteria. Active against gram-positive and gram-negative microorganisms: B. cereus, C. albicans, E. coli, P. aeruginosa, S. aureus, A. niger, S. Abony and others.
Pharmacokinetics
When applied topically, it is practically not absorbed.
Special instructions
Special instructions
Nasal discharge may turn gray or blue.
Impact on the ability to drive vehicles and machinery
No effect.
Active ingredient
Active ingredient
Silver proteinate
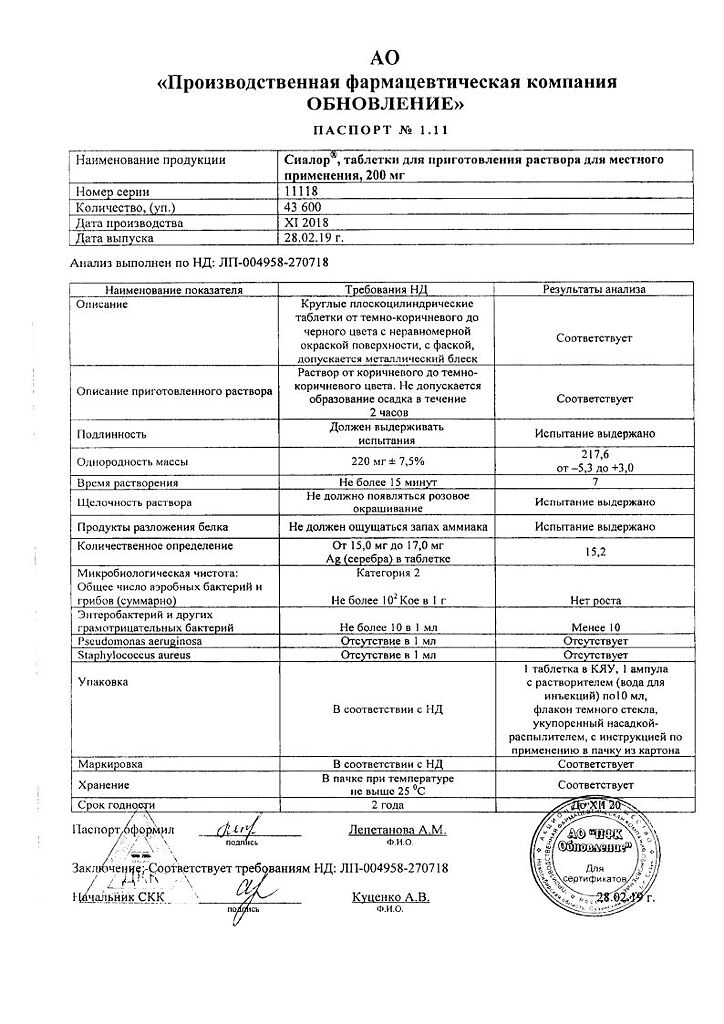
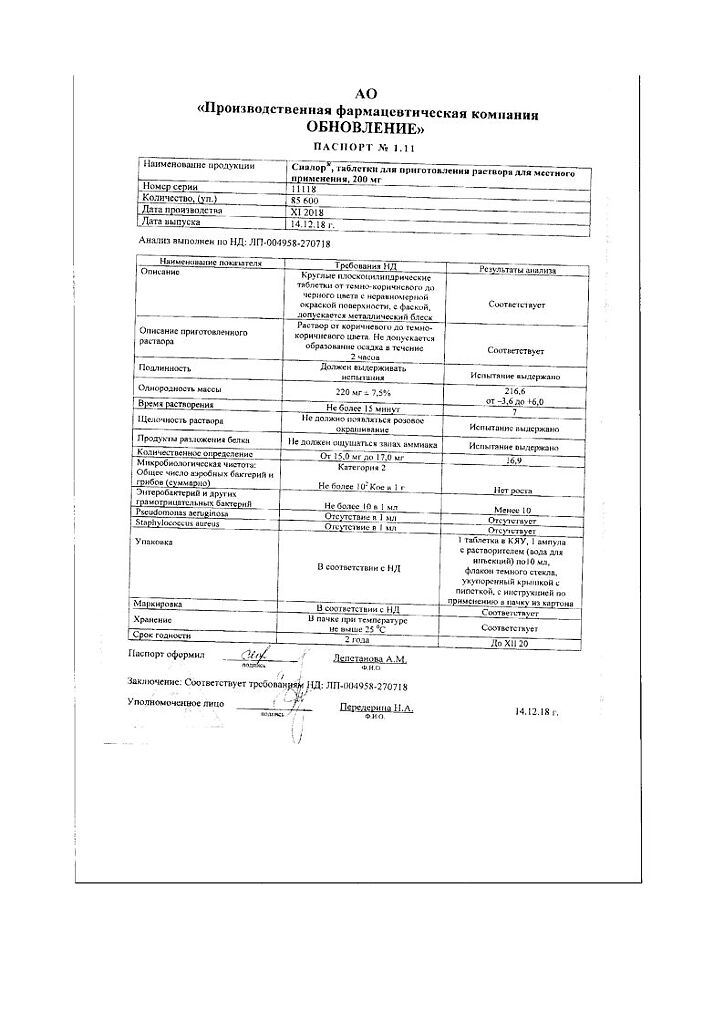
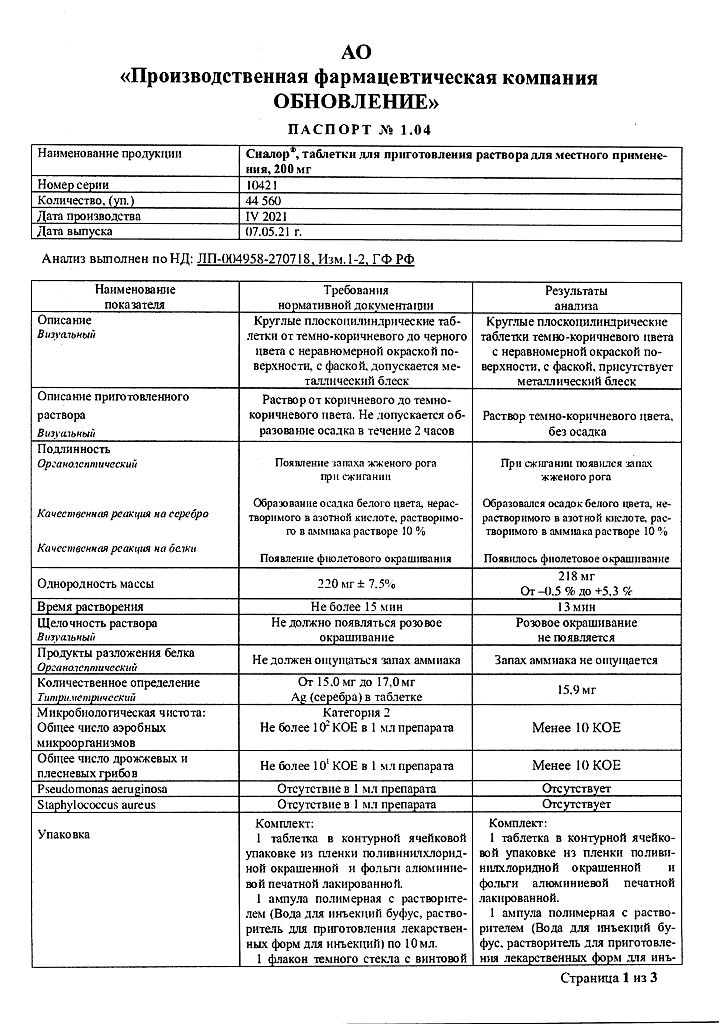
Composition
Composition
Active ingredient:
Silver proteinate – 200 mg
Excipient:
povidone K 30 – 20 mg
1 ampoule of solvent contains: water for injection.
Pregnancy
Pregnancy
The drug is contraindicated during pregnancy and nursing mothers; if it is necessary to use the drug, breastfeeding should be discontinued.
Contraindications
Contraindications
Hypersensitivity to the drug, pregnancy, lactation, atrophic rhinitis, children under 3 years of age.
Side Effects
Side Effects
Mild irritation of the mucous membrane, burning, itching, allergic reactions (urticaria, atopic dermatitis, Quincke’s edema, anaphylactic shock).
If you experience side effects listed in the instructions or they get worse, or you notice any other side effects not listed in the instructions, please contact your doctor.
Interaction
Interaction
Pharmacodynamic, pharmacokinetic interaction: not studied.
Pharmaceutical interaction: salts of zinc, copper, lead, silver, mercury, iron, aluminum form insoluble precipitates with the drug solution; the drug solution is inactivated by alkaloid salts and organic bases (epinephrine).
If you are using the above or other medications (including over-the-counter medications), consult your doctor before using the drug.
Overdose
Overdose
With the recommended method of use, an overdose is unlikely.
Symptoms: possible increased side effects. If accidentally swallowed, a large amount of the drug may cause irritation of the gastrointestinal tract.
Treatment: in case of severe irritation, burning, itching, rinse the mucous membranes with plenty of water for 15 minutes. If symptoms persist, consult a doctor.
Treatment is symptomatic.
Storage conditions
Storage conditions
In a pack at a temperature not exceeding 25 °C.
Keep out of the reach of children.
Shelf life
Shelf life
2 years.
Do not use after expiration date.
Manufacturer
Manufacturer
Update of PFC JSC, Russia
Additional information
| Shelf life | 2 years. Do not use after the expiration date. |
|---|---|
| Conditions of storage | In the package at a temperature not exceeding 25 ° C. . |
| Manufacturer | Update PFC AO, Russia |
| Medication form | nasal spray |
| Brand | Update PFC AO |
Related products
Buy Sialor, set 2% 10 ml with sprayer with delivery to USA, UK, Europe and over 120 other countries.