No products in the cart.
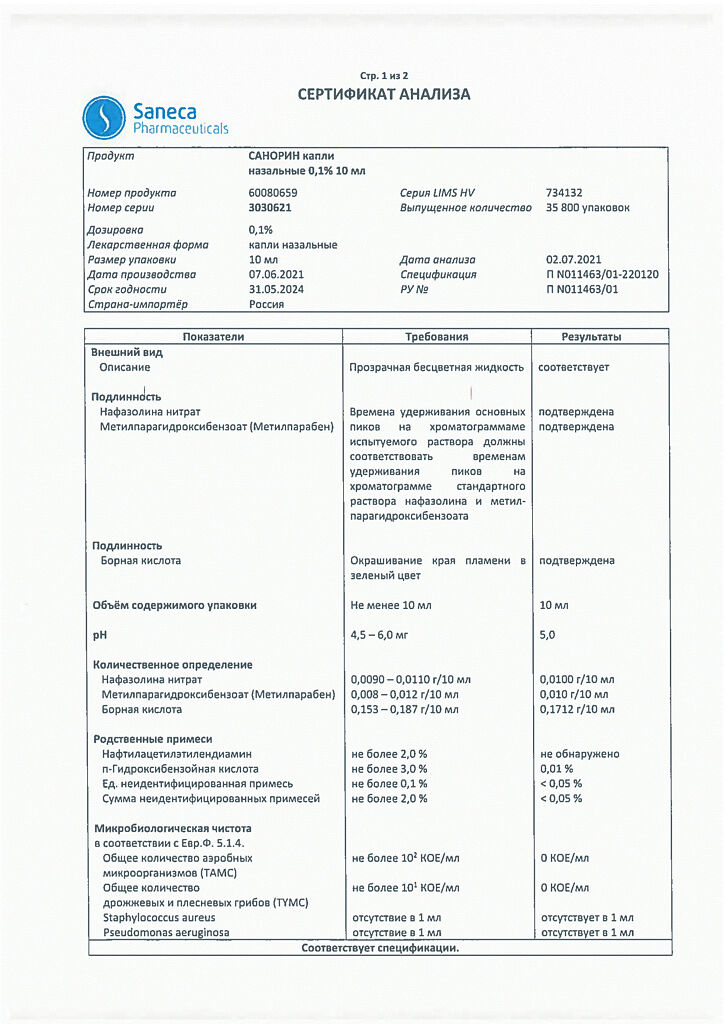
Sanorin, drops 0.1% 10 ml
€5.85 €5.12
Description
Pharmacotherapeutic group:
An anticongestive agent – alpha-adrenomimetic.
ATX code: R01AA08.
Pharmacological properties
< em>Pharmacodynamics
Nafazolin refers to alpha< sub>2-adrenomimetics with a direct stimulatory effect on alpha-adrenoreceptors of the sympathetic nervous system. When administered intranasally it has a rapid, pronounced and sustained vasoconstrictor effect on the vessels of the nasal mucous membranes, nasopharynx and sinus cavities – it reduces swelling and hyperemia, thereby improving nasal passageway and facilitating nasal breathing. At the same time the patency of the eustachian tubes is restored. The therapeutic effect usually occurs within 5 minutes after drug administration and lasts for 4-6 hours.
< em>Pharmacokinetics< /strong>
Data regarding distribution, metabolism and elimination of naphazoline in humans are not available.
Indications
Indications
Active ingredient
Active ingredient
Composition
Composition
How to take, the dosage
How to take, the dosage
Intranasally.
Adults and children over 15 years of age:
1 to 3 drops of 0.1% SANORIN® solution in each nasal passage 3-4 times daily.
Children 2-15 years old:
1-2 drops of 0.05% SANORIN® solution into each nasal passage 2-3 times a day at least 4 hours apart.
Apply for a short time, not more than 1 week in adults and not more than 3 days in children.
If nasal breathing becomes easier, use of SANORIN® can be ended earlier. Reapplication is possible after a few days.
In case of nasal bleeding, a cotton swab moistened with 0.05% SANORIN® solution may be placed in the nasal passage.
In rhinoscopy to prolong surface anesthesia: 2-4 drops of 0.1% solution with 1 ml of anesthetic.
The drug is injected into each nasal passage with the head slightly tilted back. When injecting into the left nasal passage, the head should be tilted right, and when injecting into the right nasal passage – left.
Interaction
Interaction
Special Instructions
Special Instructions
Caution should be exercised during general anesthesia with the use of anesthetics that increase myocardial sensitivity to sympathomimetics (halothane).
In rare cases, ricochet hyperemia and swelling of the nasal mucosa develop.
It may have a resorptive effect. Nausea, tachycardia, headache, irritability, increased sweating, allergic reactions, rash, increased blood pressure are extremely rare due to irritation of the sympathetic nervous system and general effects on the body.
Influence on driving and other machinery
The drug does not affect activities that require increased attention and reaction (driving vehicles, maintenance of mechanisms, high-altitude work).
Synopsis
Synopsis
Contraindications
Contraindications
– increased sensitivity to the drug components;
– chronic rhinitis;
– atrophic rhinitis;
– closed-angle glaucoma;
– severe eye disease;
– arterial hypertension;
– pronounced atherosclerosis;
– tachycardia;
– hyperthyroidism;
– diabetes mellitus;
– simultaneous use of monoamine oxidase inhibitors and the period up to 14 days after the end of their use.
SANORIN® 0,05% solution is contraindicated in children under 2 years.
SANORIN® 0,1% solution is contraindicated in children under 15 years.
With caution
Pregnancy, lactation, coronary heart disease (angina pectoris), prostatic hyperplasia, pheochromocytoma.
Side effects
Side effects
Overdose
Overdose
Pregnancy use
Pregnancy use
Similarities
Similarities
Additional information
| Shelf life | 3 years. Shelf life of the opened bottle is 4 weeks. Do not use after the expiration date |
|---|---|
| Conditions of storage | At the temperature not more than 25 oC in the original package (bottle in the package). Keep out of reach of children. |
| Manufacturer | Saneka Pharmaceuticals a.s., Slovakia |
| Medication form | nasal drops |
| Brand | Saneka Pharmaceuticals a.s. |
Other forms…
Related products
Buy Sanorin, drops 0.1% 10 ml with delivery to USA, UK, Europe and over 120 other countries.