No products in the cart.
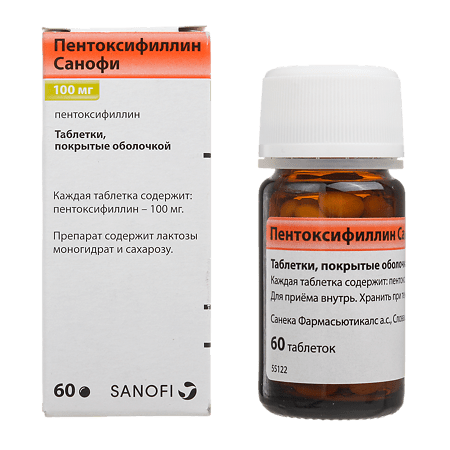
Sanofi Pentoxifylline, 100 mg 60 pcs
€11.20 €9.00
Description
Pentoxifylline improves microcirculation and rheological properties of blood.
The mechanism of action is associated with inhibition of phosphodiesterase enzyme and increase of cyclic adenosine monophosphate (cAMP) in platelets and adenosine triphosphate (ATP) in erythrocytes with simultaneous saturation of energy potential, which in turn leads to vasodilation, reduction of total peripheral vascular resistance (TPR), increase of stroke and minute blood volume without significant changes in heart rate (HR).
Pentoxifylline reduces blood viscosity, increases erythrocyte membrane elasticity (due to influence on pathologically changed erythrocyte deformability), reduces erythrocyte, platelet and neutrophil aggregation, reduces blood fibrinogen content, reduces leukocyte adhesiveness to vascular endothelium, reduces leukocyte stimulation and, as a result, endothelium destruction.
It improves microcirculation in the zones of impaired blood circulation.
In occlusive diseases of peripheral arteries (“intermittent” claudication) leads to extension of walking distance, elimination of night cramps of the calf muscles and pain at rest.
Indications
Indications
Active ingredient
Active ingredient
Composition
Composition
How to take, the dosage
How to take, the dosage
The tablets are taken orally, without chewing, at regular intervals, at the same time, preferably during or after a meal, with plenty of water and without chewing.
The drug is started at 200 mg (2 tablets) 3 times daily for the first week. If there is a rapid decrease in BP and symptoms of gastrointestinal or central nervous system irritation, the initial dose is reduced to 100 mg (1 tablet) 3 times daily.
In case of course treatment, 100 mg (1 tablet) 3 times a day is prescribed.
The maximum daily dose is 1200 mg per day.
Patients with low blood pressure and those who are in risk group due to possible sharp decrease of BP (patients with severe form of CHD or with hemodynamically significant stenosis of cerebral vessels) should start treatment with Sanofi Pentoxifylline in small doses with subsequent gradual increase of daily dose.
The peripheral arterial occlusive disease stage II (“intermittent claudication”)
The daily dose for this category of patients is 1200 mg daily, preferably as slow-release tablets of 400 mg 3 times daily or 600 mg 2 times daily.
Patients with hepatic impairment
Caution should be exercised when using Sanofi Pentoxifylline in patients with severe hepatic impairment; possible dose reduction should be performed taking into account individual tolerance of the drug.
Patients with impaired renal function
Caution should be exercised when administering Sanofi Pentoxifylline in patients with CK values below 30 ml/min. The dose of Sanofi Pentoxifylline may be decreased by 50-70%.
Children and adolescents
The use of the drug Pentoxifylline Sanofi in patients under the age of 18 years is contraindicated. Safety and efficacy of the use of the drug Pentoxifylline Sanofi in this age group has not been established.
Interaction
Interaction
With hypotensive agents
Sanofi Pentoxifylline may increase the BP decrease when used simultaneously with hypotensive agents (e.g., ACE inhibitors) or other drugs with potential hypotensive effects (e.g., nitrates).
With medicinal products affecting the clotting system
Sanofi Pentoxifylline may increase the effect of medicinal products affecting the clotting system (direct and indirect anticoagulants, thrombolytics, antibiotics such as cephalosporins).
When pentoxifylline and indirect anticoagulants (vitamin K antagonists) are used together in post-marketing studies there have been cases of increased anticoagulant effect (risk of bleeding). Therefore, at the start of administration of Sanofi Pentoxifylline or when changing the dose, it is recommended to monitor the severity of anticoagulant effect in patients taking this combination of drugs (e.g., regular monitoring of the international normalized ratio (INR)).
With cimetidine
Cimetidine may increase plasma concentrations of pentoxifylline and active metabolite I (risk of adverse reactions).
With other xanthines
Co-administration with other xanthines may lead to excessive jitters.
With hypoglycemic agents (insulin and oral hypoglycemic agents)
The hypoglycemic effects of insulin or oral hypoglycemic agents may be increased by concomitant use with Sanofi’s Pentoxifylline (increased risk of hypoglycemia). Strict monitoring of such patients is necessary, including regular glycemic control.
With theophylline
Some patients with concomitant administration of Sanofi’s Pentoxifylline and theophylline have increased plasma concentrations of theophylline. This may further increase the frequency of adverse reactions associated with theophylline.
With ciprofloxacin
Some patients with concomitant use of Sanofi Pentoxifylline and ciprofloxacin have increased plasma concentrations of pentoxifylline. This may subsequently lead to an increase or intensification of adverse reactions associated with the use of this combination.
With valproic acid
When used together, Sanofi’s Pentoxifylline may increase the effects of valproic acid.
With platelet aggregation inhibitors
. When pentoxifylline is used simultaneously with platelet aggregation inhibitors (clopidogrel, eptifibatide, tirofiban, epoprostenol, iloprost, abciximab, anagrelide, NSAIDs [except selective cyclooxygenase-2 inhibitors], acetylsalicylic acid, ticlopidine, dipyridamole) a potential additive effect may develop, increasing the risk of bleeding. Therefore, due to the risk of bleeding, caution should be exercised when using Sanofi Pentoxifylline concomitantly with the above platelet aggregation inhibitors.
Special Instructions
Special Instructions
The treatment should be carried out under control of BP.
In patients with diabetes mellitus taking hypoglycemic agents, administration of high doses of Sanofi Pentoxifylline may cause significant hypoglycemia (dose adjustment is required).
When Pentoxifylline Sanofi is prescribed concomitantly with anticoagulants, the clotting system should be monitored closely.
In patients who have recently had surgery, systematic monitoring of hemoglobin and hematocrit is necessary.
Patients with low and unstable blood pressure should have the dose of Sanofi’s Pentoxifylline adjusted individually.
In elderly patients a reduction in the dose of Sanofi’s Pentoxifylline may be required (increased bioavailability and decreased excretion rate).
The safety and efficacy of Sanofi’s Pentoxifylline in children have not been adequately studied. Caution should be exercised when using Sanofi Pentoxifylline in patients with severe hepatic dysfunction. Taking into account the risk of drug cumulation and increased risk of side effects, dose reduction should be carried out taking into account individual tolerance. Patients with significant impairment of renal function (CK below 30 ml/min) when taking Sanofi Pentoxifylline should be closely monitored by a physician.
If patients experience retinal hemorrhages while using the drug, the drug should be discontinued immediately.
Smoking may decrease the therapeutic effectiveness of Sanofi Pentoxifylline.
The drug Pentoxifylline Sanofi contains lactose and sucrose; therefore patients with intolerance to fructose, lactose, lactase, sucrose/isomaltase deficiency, glucose-galactose malabsorption are contraindicated.
Influence on ability to drive vehicles and mechanisms
No adverse effect of the drug Pentoxifylline Sanofi on the ability to drive vehicles and mechanisms has been noted. However, caution must be exercised when driving motor transport and doing activities requiring high concentration and quick psychomotor reactions due to possible dizziness and visual impairment when using Pentoxifilin Sanofi.
Contraindications
Contraindications
With caution
Perform with caution in patients with significant decreased blood pressure (BP) (risk of further BP decrease) and hemodynamically significant heart rhythm disorders; chronic heart failure (CHF); severe hepatic impairment (risk of cumulation and increased risk of side effects); renal impairment (creatinine clearance (CQ) less than 30 ml/min) (risk of cumulation and increased risk of side effects); gastric and duodenal ulcer disease; with increased tendency to bleeding, e.g., as a result of using indirect anticoagulants [vitamin K antagonists] or in disorders of the blood clotting system (risk of more severe bleeding); after recent surgical intervention (risk of bleeding); with proliferative diabetic retinopathy.
. Caution should be exercised when concomitant use of Sanofi Pentoxifylline with platelet aggregation inhibitors (clopidogrel, eptifibatide, tirofiban, epoprostenol, iloprost, abciximab, anagrelide, NSAIDs [except selective cyclooxygenase-2 inhibitors], acetylsalicylic acid, ticlopidine, dipyridamole), hypoglycemic agents (insulin and hypoglycemic agents for oral administration), ciprofloxacin and theophylline.
Side effects
Side effects
When using Sanofi’s Pentoxifylline, the following side effects are possible, which are divided into systemic organ classes according to the classification of the Medical Dictionary of Regulatory Affairs (MeddDRA). The WHO classification was used to indicate the frequency of side effects: very frequent (â¥10%); frequent (â¥1% and < 10%); infrequent (â¥0.1% and < 1%); rare (â¥0.01% and < 0.1%); very rare (< 0.01%); frequency unknown (the frequency of side effect cannot be determined from available data).
Nervous system disorders: frequency unknown – headache, dizziness, seizures, aseptic meningitis.
Psychiatric disorders: frequency is unknown – agitation, sleep disturbances, anxiety.
Cardiac disorders: frequency unknown – tachycardia, arrhythmia, angina pectoris, decreased BP.
Vascular disorders: frequency is unknown – “rushes” of blood to the skin, bleeding (including bleeding from the blood vessels of the skin, mucous membranes, gastrointestinal bleeding, nose bleeding).
Skin and subcutaneous tissue disorders: frequency unknown – skin itching, skin rash, erythema (skin redness), urticaria, edema, increased nail fragility.
Gastrointestinal disorders: frequency unknown – anorexia, bowel atony, epigastric discomfort, abdominal bloating (feeling of full stomach), vomiting, diarrhea, dry mouth, constipation, hyper-salivation (increased salivation).
Visual disorders: frequency is unknown – visual impairment, scotoma.
Blood and lymphatic system disorders: frequency unknown – thrombocytopenia, leukopenia/neutropenia, pancytopenia, hypofibrinogenemia.
Immune system disorders: frequency unknown – anaphylactic/anaphylactoid reactions, angioedema, anaphylactic shock, bronchospasm.
Hepatic and biliary tract disorders: frequency unknown – intrahepatic cholestasis, increased alanine aminotransferase (ALT) and aspartate aminotransferase (ACT) activity, increased alkaline phosphatase activity.
Overdose
Overdose
Symptoms: dizziness, nausea, “coffee grounds” type vomiting, marked BP decrease, tachycardia, arrhythmia, redness of the skin, loss of consciousness, chills, areflxia, tonic-clonic convulsions.
In case of the above disorders it is necessary to consult a doctor urgently.
Treatment: treatment is symptomatic. If the first signs of overdose occur (sweating, nausea, cyanosis) the drug administration should be stopped immediately. If the drug has been taken recently, measures aimed at preventing further absorption of the drug by eliminating it (gastric lavage) or slowing down absorption (e.g., taking activated charcoal) should be provided. Particular attention should be paid to maintaining BP and respiratory function. Diazepam should be given if seizures occur.
A specific antidote is not known.
Similarities
Similarities
Additional information
| Shelf life | 5 years. Do not use after the expiration date stated on the package. |
|---|---|
| Conditions of storage | Store at temperatures under 25 ° C. Keep out of reach of children. |
| Manufacturer | Zentiva k.s., Czech Republic |
| Medication form | pills |
| Brand | Zentiva k.s. |
Other forms…
Related products
Buy Sanofi Pentoxifylline, 100 mg 60 pcs with delivery to USA, UK, Europe and over 120 other countries.