No products in the cart.
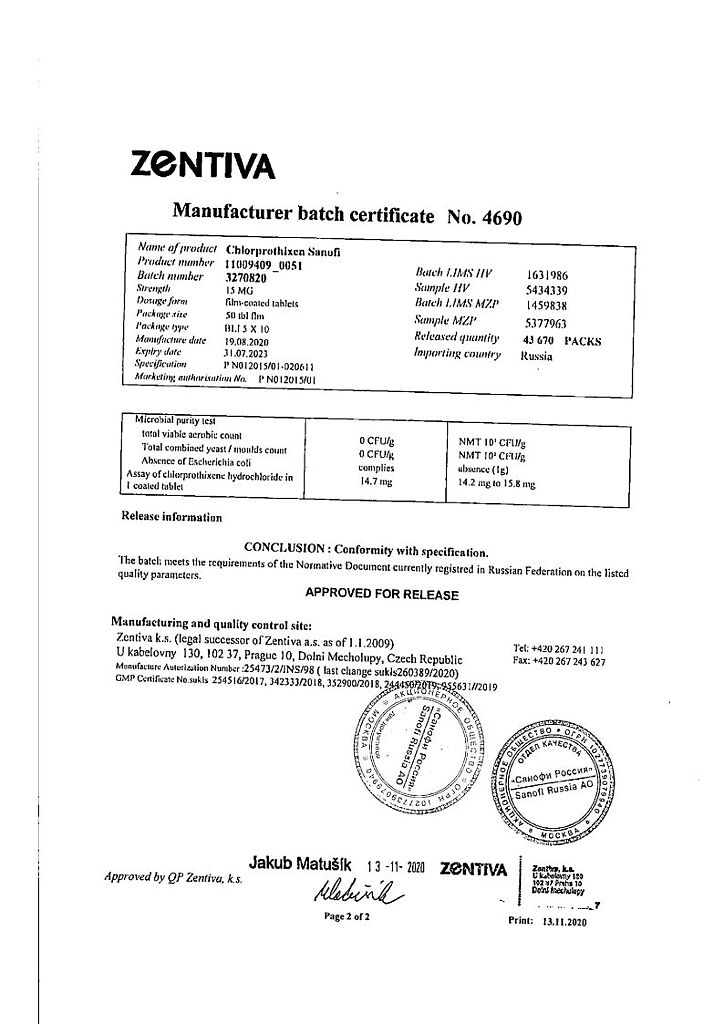
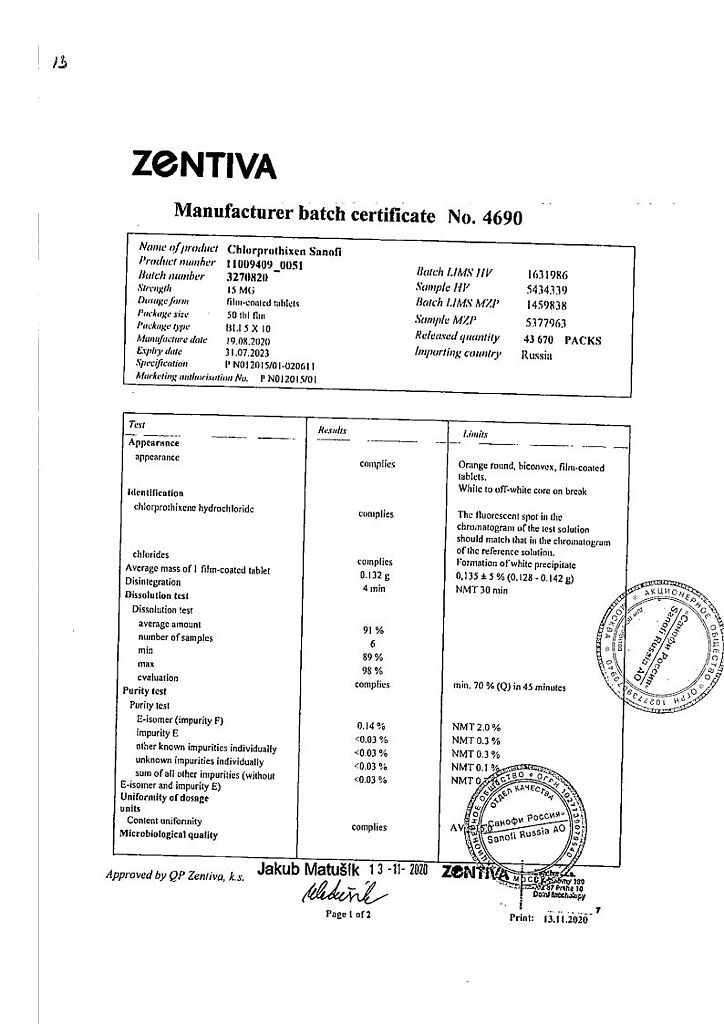
Sanofi Chlorprotixen, 15 mg 50 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
The antipsychotic action of Chlorprotixen is associated with its blocking effect on dopamine receptors. Blockade of these receptors is also associated with antiemetic and analgesic properties of the drug. Chlorprotixen is able to block 5-NT2 receptors, α1-adrenoreceptors and H1-histamine receptors, which determines its adrenoblocking hypotensive and antihistamine properties.
Pharmacokinetics
The bioavailability of chlorprotixen with oral administration is approximately 12%. Chlorprotixen is rapidly absorbed from the intestine, Cmax in serum is reached after 2 hours. T1/2 is about 16 h. Chlorprotixene penetrates the placental barrier and is excreted in small amounts with breast milk. Metabolites have no neuroleptic activity and are excreted with feces and urine.
Indications
Indications
Chlorprothixene is a sedative antipsychotic with a wide range of indications, which include:
psychoses, including schizophrenia and manic states, occurring with psychomotor agitation, agitation and anxiety;
“hangover” withdrawal syndrome in alcoholism and drug addiction;
hyperactivity, irritability, agitation, confusion in elderly patients;
behavioral disorders in children;
depressive states, neuroses, psychosomatic disorders;
insomnia;
pain (in combination with analgesics).
Pharmacological effect
Pharmacological effect
The antipsychotic effect of Chlorprothixene is associated with its blocking effect on dopamine receptors. The antiemetic and analgesic properties of the drug are also associated with the blockade of these receptors. Chlorprothixene is able to block 5-HT2 receptors, α1 adrenergic receptors, as well as H1 histamine receptors, which determines its adrenergic blocking hypotensive and antihistamine properties.
Pharmacokinetics
The bioavailability of chlorprothixene when taken orally is about 12%. Chlorprothixene is rapidly absorbed from the intestine, Cmax in the blood serum is reached after 2 hours. T1/2 is about 16 hours. Chlorprothixene penetrates the placental barrier and is excreted in small quantities in breast milk. Metabolites do not have neuroleptic activity and are excreted in feces and urine.
Special instructions
Special instructions
Chlorprothixene should be prescribed with caution to patients suffering from epilepsy, parkinsonism, with severe cerebral atherosclerosis, with a tendency to collapse, with severe cardiovascular and respiratory failure, with severe impaired liver and kidney function, diabetes mellitus, prostate hypertrophy.
The use of Chlorprothixene may lead to a false positive result when conducting an immunobiological urine test for pregnancy, a false increase in the level of bilirubin in the blood, and a change in the QT interval on the electrocardiogram.
During treatment with the drug, it is recommended to refrain from drinking alcoholic beverages and avoid increased insolation.
Impact on the ability to drive vehicles and operate machinery
Taking Chlorprothixene has a negative effect on activities that require a high speed of mental and physical reactions (for example, driving vehicles, servicing machines, working at heights, etc.).
Active ingredient
Active ingredient
Chlorprothixene
Composition
Composition
1 film-coated tablet contains:
active ingredient:
chlorprothixene hydrochloride 15 and 50 mg,
excipients:
corn starch,
lactose monohydrate,
sucrose,
calcium stearate,
talc;
shell composition:
hypromellose 2910/5,
macrogol 6000,
macrogol 300,
talc,
aluminum varnish based on sunset yellow dye.
Pregnancy
Pregnancy
Chlorprothixene should, if possible, not be prescribed to pregnant women or during breastfeeding.
Contraindications
Contraindications
hypersensitivity to the components of Chlorprothixene;
CNS depression of any origin (including those caused by alcohol, barbiturates or opiates);
comatose states;
vascular collapse;
diseases of the hematopoietic organs;
pheochromocytoma.
Side Effects
Side Effects
Drowsiness, tachycardia, dry mouth, increased sweating, difficulty in accommodation. These side effects, which usually occur early in therapy, often disappear as therapy continues.
Orthostatic hypotension may occur, especially when using Chlorprothixene in high dosages.
Dizziness, dysmenorrhea, skin rashes, and constipation are rare. Extrapyramidal symptoms are especially rare.
Isolated cases of a decrease in the convulsive threshold, the occurrence of transient benign leukopenia and hemolytic anemia have been described.
With long-term use, especially in high doses, the following may be observed: cholestatic jaundice, galactorrhea, gynecomastia, decreased potency and/or libido, increased appetite, increased body weight.
Interaction
Interaction
The inhibitory effect of chlorprothixene on the central nervous system may be enhanced when taken simultaneously with ethanol and ethanol-containing drugs, anesthetics, opioid analgesics, sedatives, hypnotics, and neuroleptics.
The anticholinergic effect of chlorprothixene is enhanced by the simultaneous use of anticholinergic, antihistamine and antiparkinsonian drugs.
The drug enhances the effect of antihypertensive drugs.
The simultaneous use of chlorprothixene and adrenaline can lead to arterial hypotension and tachycardia.
The use of chlorprothixene leads to a decrease in the threshold of convulsive activity, which requires additional adjustment of the dose of antiepileptic drugs in patients with epilepsy.
The ability of chlorprothixene to block dopamine receptors reduces the effectiveness of levodopa.
Extrapyramidal disorders may occur with the simultaneous use of phenothiazines, metoclopramide, haloperidol, and reserpine.
Overdose
Overdose
Symptoms. Drowsiness, hypo- or hyperthermia, extrapyramidal symptoms, convulsions, shock, coma.
Treatment. Symptomatic and supportive. Gastric lavage should be performed as quickly as possible, and the use of a sorbent is recommended.
Measures should be taken to maintain the functioning of the respiratory and cardiovascular systems.
Adrenaline should not be used because this may lead to a subsequent decrease in blood pressure. Seizures can be treated with diazepam, and extrapyramidal disorders with biperiden.
Storage conditions
Storage conditions
In a place protected from light, at a temperature not exceeding 25°C.
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Zentiva k.s., Czech Republic
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a place protected from light, at a temperature not exceeding 25°C. |
| Manufacturer | Zentiva k.s., Czech Republic |
| Medication form | pills |
| Brand | Zentiva k.s. |
Other forms…
Related products
Buy Sanofi Chlorprotixen, 15 mg 50 pcs with delivery to USA, UK, Europe and over 120 other countries.