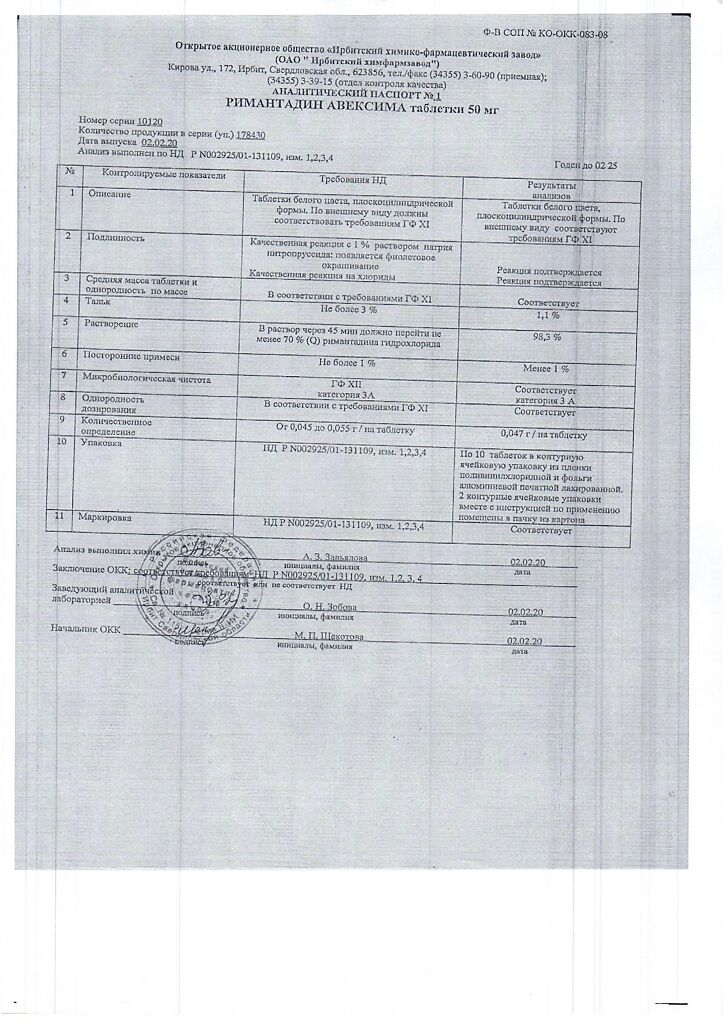
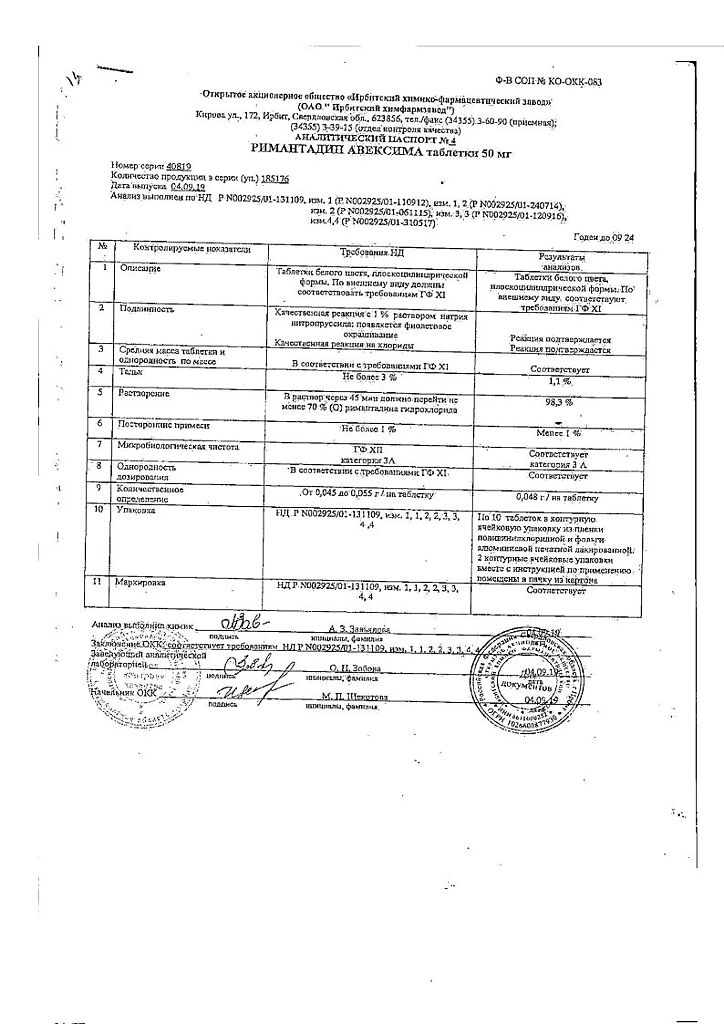
-
×


-
×


-
×


-
×


-
×


-
×


-
×


-
×


-
×


-
×


Subtotal: €197.14