No products in the cart.
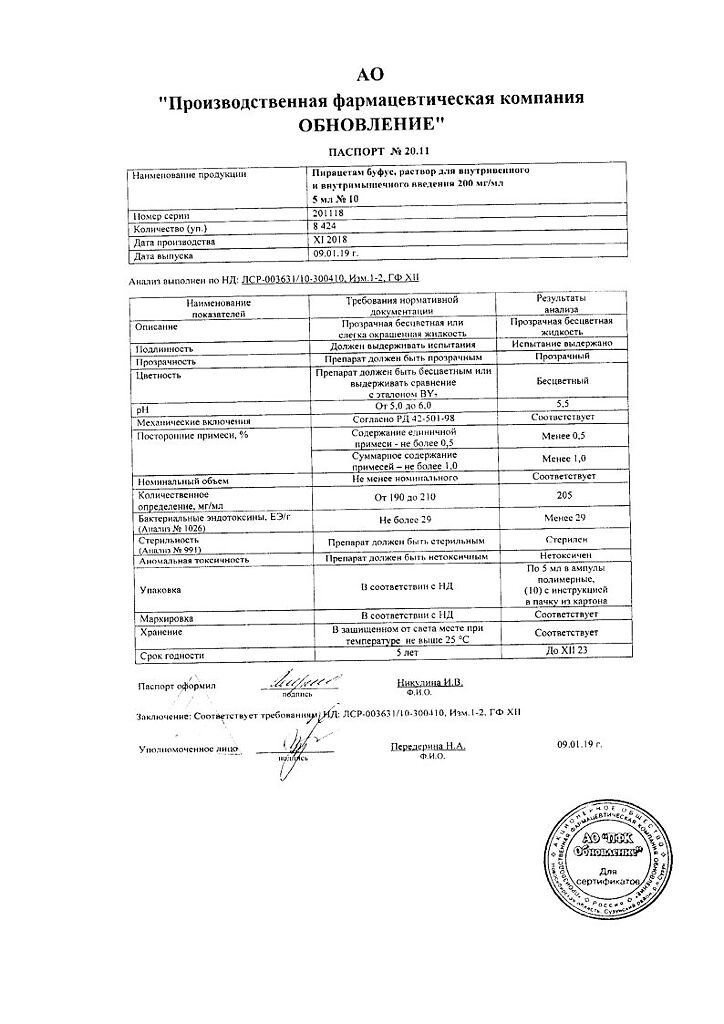
Piracetam bufus Reneval, 200 mg/ml 5 ml 10 pcs
€3.88 €3.53
Description
Pharmacotherapeutic group: Nootropic agent
Pharmacological action
Notropic agent. It has a positive effect on metabolic processes and blood circulation of the brain. Increases glucose utilization, improves metabolic processes, improves microcirculation in ischemic areas, and inhibits activated platelet aggregation. It has a protective effect in brain damage caused by hypoxia, intoxication, electroshock. It improves integrative activity of the brain. It has no sedative and psychostimulant effect.
Pharmacokinetics
Indications
Indications
Symptomatic treatment of intellectual-mnestic disorders in the absence of an established diagnosis of dementia;
reducing the manifestations of cortical myoclonus in patients sensitive to piracetam, both as monotherapy and as part of complex therapy (in order to determine sensitivity to piracetam in a particular case, a trial course of treatment can be carried out).
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: Nootropic drug
Pharmacological action
Nootropic drug. It has a positive effect on metabolic processes and blood circulation in the brain. Increases glucose utilization, improves metabolic processes, improves microcirculation in ischemic areas, inhibits aggregation of activated platelets. It has a protective effect against brain damage caused by hypoxia, intoxication, and electric shock. Improves integrative brain activity. Does not have a sedative or psychostimulating effect.
Pharmacokinetics
Special instructions
Special instructions
Use with caution in patients with severe hemostatic impairment, during major surgical operations and severe bleeding; with renal failure.
Continuous monitoring of renal function indicators is recommended.
If sleep disturbances occur, it is recommended to stop taking piracetam in the evening and add this dose to the daytime dose.
Active ingredient
Active ingredient
Piracetam
Composition
Composition
1 ml of solution contains:
active substance:
piracetam – 200 mg
excipients:
sodium acetate – 1 mg
acetic acid – up to pH 5.8
water for injection – up to 1 ml.
Pregnancy
Pregnancy
Animal studies have not shown direct or indirect effects on pregnancy, embryo/fetal development, childbirth or postnatal development. There have been no controlled studies of the use of the drug during pregnancy. Piracetam penetrates the placental barrier.
The concentration of the drug in newborns reaches 70-90% of its concentration in the mother’s blood. Piracetam should be prescribed during pregnancy only in exceptional cases, if the benefit to the mother outweighs the potential risk to the fetus, and the clinical condition of the pregnant woman requires treatment with piracetam.
Piracetam passes into breast milk. Piracetam should not be used during breastfeeding, or breastfeeding should be discontinued while being treated with piracetam. When deciding to stop breastfeeding or refuse treatment with piracetam, the benefits of breastfeeding for the child should be weighed against the benefits of therapy for the woman.
Contraindications
Contraindications
Individual intolerance to piracetam or pyrrolidone derivatives, as well as other components of the drug.
Huntington’s chorea.
Acute cerebrovascular accident (hemorrhagic stroke).
End-stage renal failure (with creatinine clearance less than 20 ml/min).
Psychomotor agitation at the time of drug administration.
Children under 3 years of age.
With caution: impaired hemostasis; extensive surgical interventions; heavy bleeding.
Side Effects
Side Effects
From the central nervous system and peripheral nervous system: motor disinhibition (1.72%), irritability (1.13%), drowsiness (0.96%), depression (0.83%), asthenia (0.23%).
In isolated cases – headache, insomnia, agitation, imbalance, ataxia, exacerbation of epilepsy, anxiety, hallucinations, confusion.
From the cardiovascular system: increased or decreased blood pressure.
From the digestive system: in isolated cases – nausea, vomiting, diarrhea, abdominal pain (including gastralgia).
Metabolism: increase in body weight (1.29%).
On the part of the skin – dermatitis, itching, rashes.
Interaction
Interaction
The possibility of changes in the pharmacokinetics of piracetam under the influence of other drugs is low, since 90% of the drug is excreted unchanged by the kidneys.
When used concomitantly with thyroid hormones, there have been reports of confusion, irritability, and sleep disturbances.
Overdose
Overdose
A single case of the development of dyspeptic symptoms in the form of diarrhea with blood and pain in the abdominal area was registered when taking the drug orally at a daily dose of 75 g.
Apparently, this was due to the use of a large total dose of sorbitol, which was previously included in the drug for the dosage form of an oral solution. No other cases of drug overdose have been identified.
Treatment: in case of overdose, symptomatic therapy is recommended, which may include hemodialysis. There is no specific antidote.
The effectiveness of hemodialysis for piracetam is 50-60%.
Storage conditions
Storage conditions
In a place protected from light, at a temperature not exceeding 25 °C.
Keep out of the reach of children.
Shelf life
Shelf life
5 years.
Manufacturer
Manufacturer
Update of PFC JSC, Russia
Additional information
| Shelf life | 5 years. |
|---|---|
| Conditions of storage | In the light-protected place at the temperature not more than 25 °C. Keep out of reach of children. |
| Manufacturer | Update PFC AO, Russia |
| Medication form | solution |
| Brand | Update PFC AO |
Related products
Buy Piracetam bufus Reneval, 200 mg/ml 5 ml 10 pcs with delivery to USA, UK, Europe and over 120 other countries.