No products in the cart.
Pentaxime, lyophilisate 1 dose 0.5 ml
€1.00
Out of stock
(E-mail when Stock is available)
Description
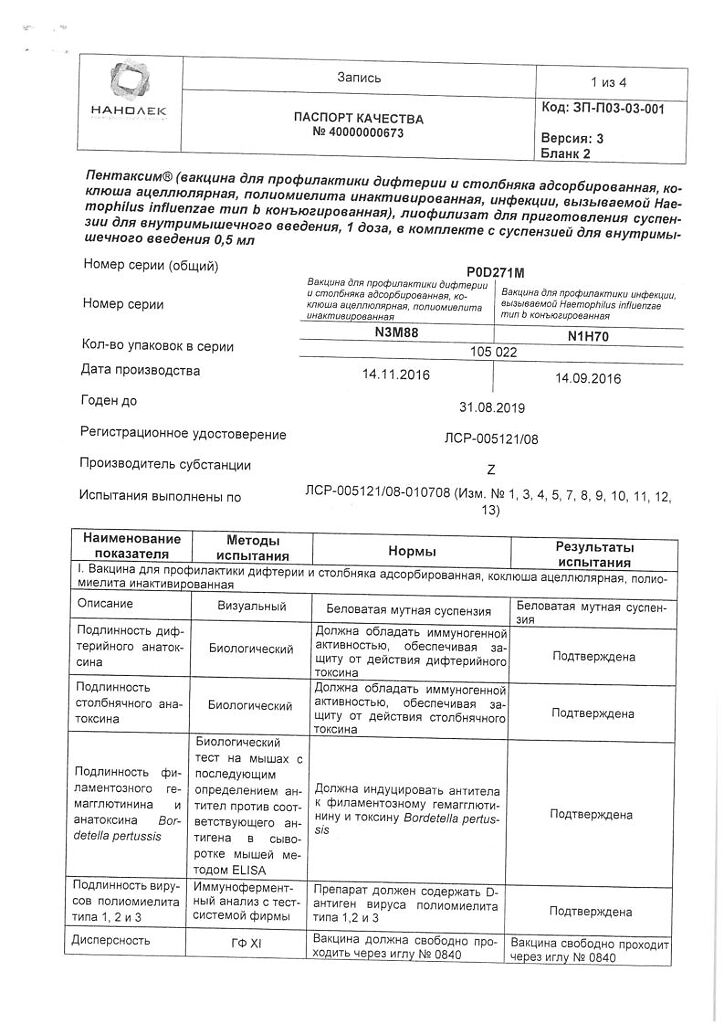
Pentaxime is a vaccine to prevent diphtheria, tetanus, pertussis, polio and diseases caused by Haemophilus influenzae type b.
Indications
Indications
Prophylaxis in children from 3 months of age:
Composition
Composition
The suspension for intravenous injection is whitish, cloudy.
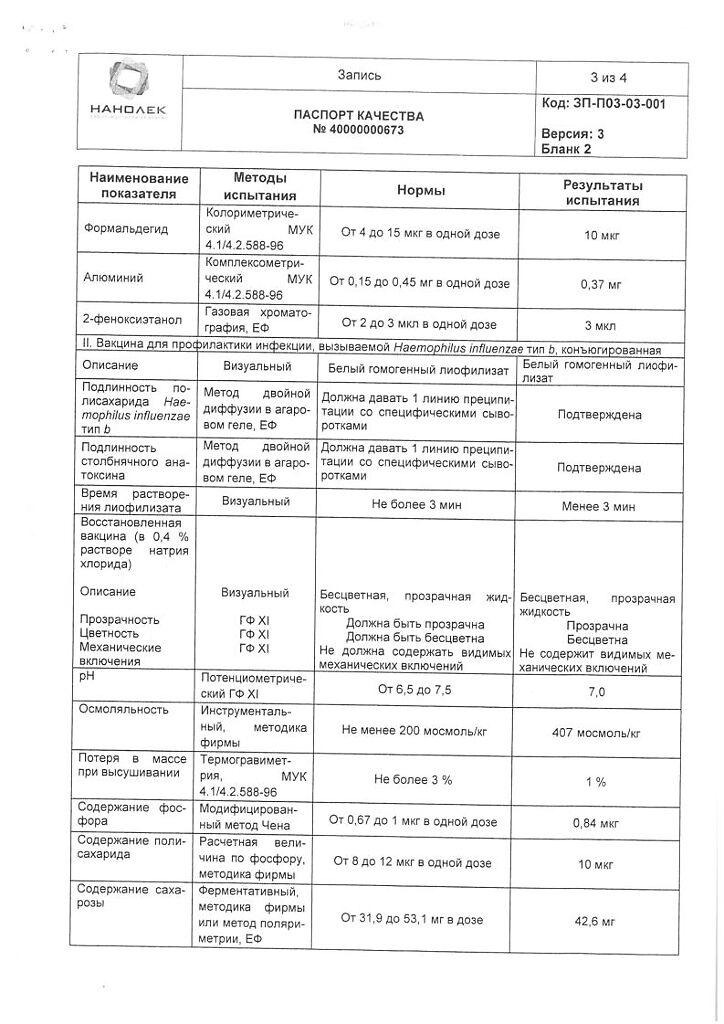
1 dosediphtheria toxoidâ¥30 IU
tetanus toxoidâ¥40 IU
pertussis toxoid25 µg
hemagglutinin filamentous25 µg
polio virus inactivated,
br>
1 type40 units D antigen polio virus inactivated,
2 type8 IU of D antigen polio virus inactivated,
3 type32 IU of D antigen
Auxiliary substances:
Aluminum hydroxide – 0.3 mg,
HANKS medium 199* – 0.05 ml,
formaldehyde – 12.5 mg,
phenoxyethanol – 2.5 µl,
acetic acid or sodium hydroxide – to pH 6.8-7.3,
d/i water – to 0.5 ml.
* – does not contain phenol red
1 dose (0.5 ml) – glass syringes (1) – contour cell packs (1) – carton packs.
How to take, the dosage
How to take, the dosage
The vaccine is administered intramuscularly in a dose of 0.5 ml, the recommended site of administration being the middle third of the anterolateral surface of the thigh. Do not inject intradermally or intravenously. Make sure that the needle has not penetrated a blood vessel before injection. For the package option with two separate needles, before preparing the vaccine, the needle should be tightly secured by rotating it a quarter of a turn relative to the syringe. To prepare the vaccine, after removing the plastic colored cap from the vial, completely inject the suspension for intramuscular administration (vaccine to prevent diphtheria, tetanus; pertussis and polio) through the needle from the syringe into the bottle with the lyophilizate (vaccine for the prevention of infection caused by Haemophilus influenzae type b).
Shake the vial without removing the syringe from it and wait for the lyophilizate to dissolve completely (no more than 3 minutes). The resulting suspension should be turbid and have a whitish tint. The vaccine should not be used if the color changes or foreign particles are present. The vaccine prepared in this way should be completely filled into the same syringe. The prepared vaccine should be given immediately.
The Pentaxime vaccine course consists of 3 injections in 1 dose (0.5 ml) every 1 -2 months, starting at 3 months of age. Revaccination is carried out.
The course of Pentaxime vaccination consists of 3 injections with one dose of vaccine (0.5 ml) at intervals of 1-2 months, starting from the age of 3 months. Revaccination is carried out by injecting 1 dose of Pentaxime at 18 months of life.
In accordance with the National calendar of preventive vaccinations of the Russian Federation the course of vaccination to prevent diphtheria, tetanus, whooping cough and poliomyelitis consists of 3 injections with an interval of 1.5 months, at the age of 3, 4.5 and 6 months respectively; the revaccination is done once at the age of 18 months.
If the vaccination schedule is violated, the subsequent intervals between administering the next dose of vaccine are not changed, including the interval before the 4th (revaccination) dose -12 months
If the first dose of Pentaxime was administered at 6-12 months, then the second dose is administered 1.5 months after the first dose, and the third dose, administered 1.5 months after the second dose, should be the diphtheria, tetanus; pertussis and polio vaccine originally presented in a syringe (i.e., without the lyophilizate diluted in the vial (Hlb)) As a revaccinating (4th dose), the usual dose of Pentaxime (with lyophilizate dilution (Hlb)) is used.
If the first dose of Pentaxime is given after 1 year of age, then the 2nd, 3rd and 4th (revaccinating) doses must use the diphtheria, tetanus, pertussis and polio vaccine originally presented in the syringe, without the lyophilizate diluted in the bottle (Hlb).
First vaccination, child’s age (full Pentaxime preparation administered)Second vaccination (after 1.5 months), administered:Third vaccination (after 1.5 months), administered:Revaccination (after 12 months), administered before 6 months Pentaxime full dose Pentaxime full dose Pentaxime 6-12 months Pentaxime full dose Pentaxime without dilution of Hlb lyophilisate in bottle Pentaxime full dose after 12 months Pentaxime without dilution of Hlb lyophilisate in bottlePentaxime without dilution of Shb lyophilisate in bottlePentaxime without dilution of Hlb lyophilisate in bottle/p>
In all cases of violation of the vaccination schedule, the doctor must be guided by the National Preventive Immunization Calendar of the Russian Federation.
Interaction
Interaction
With the exception of immunosuppressive therapy, there are no reliable data on possible reciprocal effects when used with other medications, including other vaccines.
The physician must be informed if the child has had any other medications (including over-the-counter medications) recently administered or coincided with the vaccination.
Special Instructions
Special Instructions
Pentaxime does not form immunity against infections caused by other Haemophilus influenzae serotypes, nor against meningitis of other etiologies.
The physician must be informed of all cases of adverse reactions, including those not listed in these instructions. Before each vaccination, to prevent possible allergic and other reactions, the doctor should clarify the state of health, immunization history, history of the patient and next of kin (in particular – allergological), cases of adverse effects on the previous vaccine administration. The doctor must have the medications and tools necessary for the development of a hypersensitivity reaction.
Immunosuppressive therapy or an immunodeficiency state may cause a weak immune response to the vaccine administration. In these cases, it is recommended to postpone vaccination until such therapy has ended or the disease is in remission. However, those with chronic immunodeficiencies (e.g., HIV infection) vaccination is recommended, even though the immune response may be impaired.
In cases of thrombocytopenia and other blood clotting disorders, administration of the vaccine should be done with caution because of the risk of bleeding during an IUI.
If a history of Guillain-Barré syndrome or brachial neuritis develops in response to any vaccine containing tetanus toxoid, the decision to vaccinate with Pentaxime must be carefully justified. As a rule, completion of the initial immunization (if less than 3 doses have been administered) is justified in such cases.
Contraindications
Contraindications
Side effects
Side effects
Local: soreness (usually expressed by brief crying at rest or by gentle pressure on the injection site); redness and swelling at the injection site (in 0.1-1% of cases, >5 cm in diameter). These reactions may develop within 48 hours after vaccination.
General: increased body temperature: >38°C, with a frequency of 1-10%; >39°C, with a frequency of 0.1-1%; rarely (0.01-0.1%) over 40°C. (Rectal temperature was assessed; as a rule, it was 0.6-1.1°C higher than axillary (axillary) temperature.)
In addition, irritability, somnolence, sleep disturbances, anorexia, diarrhea, vomiting, and, rarely, prolonged crying were noted.
In very rare (< 0.01%) rash, urticaria, febrile and afebrile seizures, hypotension and hypotonic-hyporeactive syndrome, anaphylactic reactions (facial edema, Quincke’s edema, shock) were noted
Rarely, after administration of vaccines containing the HIb component, edema of one or both lower extremities has been observed (with predominant edema on the limb where the vaccine was administered). Mostly edema was observed within the first few hours after the initial vaccination.
These reactions were sometimes accompanied by elevated body temperature, soreness, prolonged crying, cyanosis or changes in skin color, less often by redness, petechiae or transient purpura, elevated body temperature, rash. These reactions resolved on their own within 24 hours without any residual effects, and were not associated with any adverse events of the heart or respiratory system.
Very rarely, after administration of vaccines containing the acellular pertussis component, there have been cases of marked reactions (more than 5 cm in diameter) at the vaccine injection site, including edema spreading beyond one or both joints. These reactions appeared 24-72 hours after vaccine administration and might be accompanied by redness, increased skin temperature at the injection site, sensitivity or soreness at the injection site. These symptoms disappeared on their own within 3-5 days without any additional treatment. The probability of such reactions is believed to increase according to the number of injections of the acellular pertussis component, this probability being greater after the 4th and 5th doses of such a vaccine. The company has data that Guillain-Barré syndrome and brachial neuritis have been observed after other vaccines containing tetanus toxoid.
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | At 2-8 °C (do not freeze). Keep out of reach of children. |
| Manufacturer | Sanofi Pasteur S.A., France |
| Medication form | lyophilizate |
| Brand | Sanofi Pasteur S.A. |
Related products
Buy Pentaxime, lyophilisate 1 dose 0.5 ml with delivery to USA, UK, Europe and over 120 other countries.