No products in the cart.
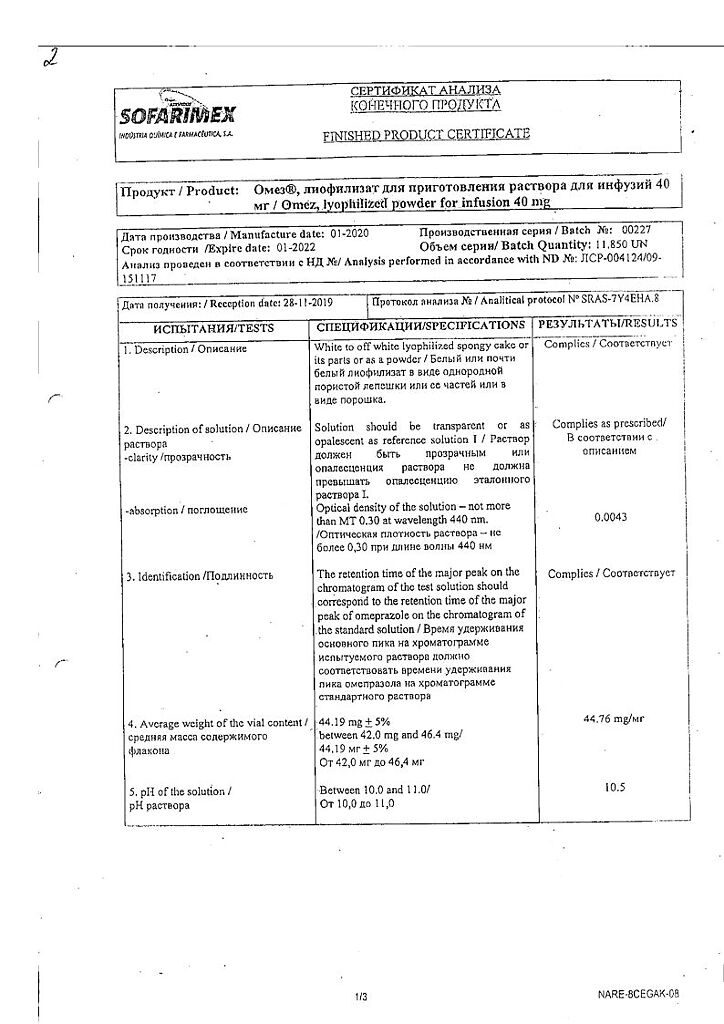
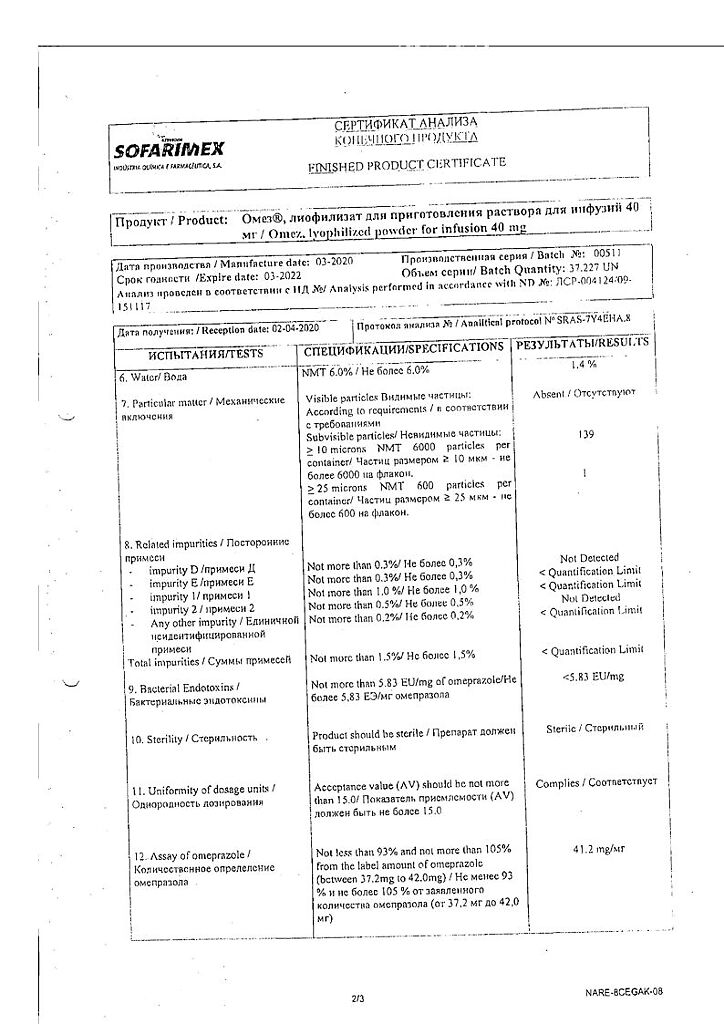
Omez, lyophilizate 40 mg
€4.82 €4.02
Out of stock
(E-mail when Stock is available)
EAN: 8901148213494
SKU: 222976
Categories: Medicine, Stomach, intestines, liver, Ulcer and gastritis
Description
Gastrointestinal infections caused by Helicobacter pylori, Heartburn, Reflux esophagitis, Peptic ulcer, Pain in the epigastric region, Nausea, Sour belching As an alternative to oral therapy if it is impossible:
- in gastric and duodenal ulcer disease (including prevention of relapse);
- in gastroesophageal reflux disease (GERD);
- with hypersecretory conditions (Zollinger-Ellison syndrome, stress ulcers of the gastrointestinal tract, polyendocrine adenomatosis, systemic mastocytosis);
- for prevention and treatment of gastric and duodenal mucosal damage caused by taking non-steroidal anti-inflammatory drugs (NSAID gastropathy): dyspepsia, mucosal erosions, peptic ulcer;
- to prevent aspiration of acidic stomach contents into the airways during general anesthesia (Mendelsohn syndrome).
.
Indications
Indications
As an alternative to oral therapy if it is not possible:
for peptic ulcers of the stomach and duodenum (including prevention of relapses);
for gastroesophageal reflux disease (GERD);
for hypersecretory conditions (Zollinger-Ellison syndrome, stress ulcers of the gastrointestinal tract, polyendocrine adenomatosis, systemic mastocytosis);
for the prevention and treatment of damage to the mucous membrane of the stomach and duodenum caused by the use of nonsteroidal anti-inflammatory drugs (NSAID gastropathy): dyspepsia, erosion of the mucous membrane, peptic ulcer;
to prevent aspiration of acidic stomach contents into the respiratory tract during general anesthesia (Mendelssohn syndrome).
Pharmacological effect
Pharmacological effect
Pharmacodynamics
Special instructions
Special instructions
If a gastric ulcer is suspected in the early stages, it is necessary to undergo an X-ray or endoscopic examination to establish the correct diagnosis and prescribe adequate treatment.
In the presence of any alarming symptoms, such as significant spontaneous weight loss, frequent vomiting, dysphagia, hematemesis or melena, as well as in the presence of a gastric ulcer (or suspected gastric ulcer), the possibility of malignancy should be excluded, since treatment with Omez® may lead to improvement of symptoms and delay diagnosis.
Patients at risk of developing osteoporosis or osteoporotic fractures should be under appropriate clinical supervision, although a causal relationship between omeprazole/esomeprazole and osteoporotic fractures has not been established.
Due to a decrease in the secretion of hydrochloric acid, the concentration of chromogranin A (CgA) increases. Increased concentrations of CgA in blood plasma may affect the results of examinations to detect neuroendocrine tumors. To prevent this effect, therapy with proton pump inhibitors should be suspended 5 days before the CgA concentration study. If during this time the CgA concentration has not returned to normal, the study should be repeated.
During treatment with omeprazole, dizziness, drowsiness, and blurred vision may occur, so care should be taken when driving vehicles and performing other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Omeprazole
Composition
Composition
Each bottle contains:
Pregnancy
Pregnancy
The use of the drug during pregnancy is possible only if the expected benefit to the mother outweighs the potential risk to the fetus.
Contraindications
Contraindications
Hypersensitivity to omeprazole, substituted benzimidazoles or other components of the drug.
Side Effects
Side Effects
The frequency of adverse drug reactions is presented in accordance with the following gradation: very often (>1/10); often (≥1/100, <1/10); uncommon (≥1/1000, <1/100); rare (≥1/10000, <1/1000); very rare (<1/10000, including isolated cases).
Interaction
Interaction
Long-term use of omeprazole at a dose of 20 mg 1 time per day in combination with caffeine, theophylline, piroxicam, diclofenac, naproxen, metoprolol, propranolol, ethanol, cyclosporine, lidocaine, quinidine and estradiol did not lead to changes in their concentrations in the blood plasma.
Overdose
Overdose
Symptoms: confusion, blurred vision, drowsiness, dry mouth, headache, nausea, tachycardia, arrhythmia.
Treatment: symptomatic. Hemodialysis is not effective enough.
Storage conditions
Storage conditions
In a place protected from light at a temperature not exceeding 25 ºС.
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Colep Consumer Products Portugal S.A., Portugal
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In the light-protected place at a temperature not exceeding 25 ºC. |
| Manufacturer | Portugal |
| Medication form | lyophilizate |
Other forms…
Related products
Buy Omez, lyophilizate 40 mg with delivery to USA, UK, Europe and over 120 other countries.