No products in the cart.
OKI, 150 ml
€22.10 €18.41
Description
Pharmacotherapeutic group
Other oral medications.
TAC code: A01AD11.
Pharmacodynamics
It has anti-inflammatory, analgesic effects. By inhibiting cyclooxygenase types I and II, inhibits the synthesis of inflammatory mediators (prostaglandins, thromboxane, etc.). Stabilizes lysosomal membranes and inhibits the release of enzymes that contribute to tissue destruction during inflammation. Inhibits the activity of neutrophils. It has anti-bradykinin activity. The drug does not have antibacterial activity.
Pharmacokinetics
A single dose of 160 mg results in plasma levels of less than 400 ng/mL, insufficient for systemic action.
Indications
Indications
Symptomatic treatment of inflammatory diseases of the upper respiratory tract – tonsillitis (sore throat), laryngitis, pharyngitis;
inflammatory diseases of the oral cavity – stomatitis, gingivitis, glossitis, aphtha, periodontopathy, chronic periodontal disease;
as an analgesic during dental procedures.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group
Other drugs for the treatment of oral diseases.
ATX code: A01AD11.
Pharmacodynamics
Has anti-inflammatory, analgesic effect. By inhibiting cyclooxygenase types I and II, it inhibits the synthesis of inflammatory mediators (prostaglandins, thromboxane, etc.). Stabilizes lysosome membranes and delays the release of enzymes that contribute to tissue destruction during inflammation. Inhibits the activity of neutrophils. Has antibradykinin activity. The drug does not have an antibacterial effect.
Pharmacokinetics
A single dose of 160 mg results in a plasma drug level of less than 400 ng/ml, which is insufficient for systemic action.
Special instructions
Special instructions
Long-term use may cause hypersensitivity reactions. In this case, you should stop using the drug and select adequate treatment methods.
When using high doses and a long course of treatment, absorbed ketoprofen can compete with other drugs that have a high affinity for plasma proteins.
The drug contains methyl parahydroxybenzoate, which can cause allergic reactions (including delayed allergic reactions).
Impact on driving vehicles and machinery
There is no data on the negative effect of the drug on the ability to drive vehicles and engage in other potentially hazardous activities that require concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Ketoprofen
Composition
Composition
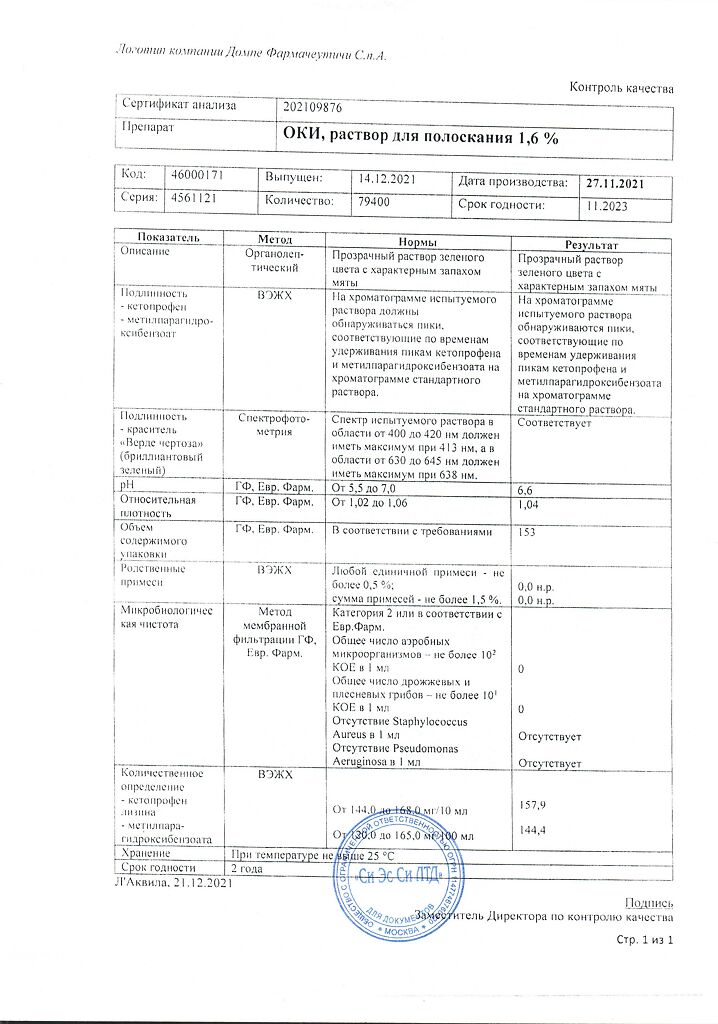
For 10 ml of solution:
Active ingredient:
ketoprofen lysine (ketoprofen lysine salt) 160.0 mg.
Excipients:
glycerol 85% 2000.0 mg,
ethanol 0.5 ml,
methyl parahydroxybenzoate 15.0 mg,
mint flavor 30.0 mg,
levomenthol 7.0 mg,
sodium saccharinate 20.0 mg,
dye “Verde Certosa” (brilliant green) 1.6 mg,
sodium hydrogen phosphate to pH 6.0-6.5,
purified water up to 10 ml.
Pregnancy
Pregnancy
NSAIDs should not be used by women from the 20th week of pregnancy due to the possible development of oligohydramnios and/or kidney pathology in newborns (neonatal renal dysfunction). Use of the drug before the 20th week of pregnancy is possible only after consultation with a doctor, if the expected benefit to the mother outweighs the potential risk to the fetus.
The use of the drug during breastfeeding is not recommended.
Contraindications
Contraindications
Hypersensitivity to ketoprofen and other components of the drug, acetylsalicylic acid or other NSAIDs, complete or incomplete combination of bronchial asthma, recurrent nasal polyposis or paranasal sinuses and intolerance to acetylsalicylic acid and other NSAIDs (including a history), childhood (up to 12 years), pregnancy over 20 weeks.
With caution
Erosive and ulcerative lesions of the gastrointestinal tract (exacerbation), Crohn’s disease, diverticulitis, peptic ulcer, hemophilia and other bleeding disorders, chronic heart failure, bronchial asthma, old age, pregnancy (up to the 20th week).
Side Effects
Side Effects
Very rare cases of local irritation and angioedema (swelling of the lips, face, throat or tongue) have been reported, especially in patients with hypersensitivity to NSAIDs. There are no known cases of systemic side effects.
Interaction
Interaction
Not detected.
Overdose
Overdose
Currently, no cases of overdose with OCI have been reported.
Storage conditions
Storage conditions
Store at a temperature not exceeding 25 oC.
Keep out of the reach of children!
Shelf life
Shelf life
Shelf life: 3 years.
Do not use the drug after the date indicated on the package.
Manufacturer
Manufacturer
Dompe Pharmaceutici S.p.A., Italy
Additional information
| Shelf life | Shelf life 3 years. Do not use the drug after the date shown on the package. |
|---|---|
| Conditions of storage | Store at temperatures under 25 oC. Keep out of reach of children! |
| Manufacturer | Dompe Pharmaceutici S.p.A., Italy |
| Medication form | gargle solution |
| Brand | Dompe Pharmaceutici S.p.A. |
Related products
Buy OKI, 150 ml with delivery to USA, UK, Europe and over 120 other countries.