No products in the cart.
Oftagel, eye gel 0.25% 10 g
€11.57 €9.64
Description
Oftagel has a good moisturizing effect with prolonged contact with the cornea.
Pharmacodynamics
The polymeric chains of carboxyvinyl polymer (polyacrylate) interact with the mucin layer on the corneal epithelium. The non-ionized carboxylic acid residues in the polymer form hydrogen bonds with mucin molecules. Carbomer also contains more ionized sites that retain water around the molecule through electrostatic forces. In addition to bioadhesion, it increases tear viscosity by forming a protective moisturizing film on the corneal surface. It thickens the mucin and water layers of the tear film. In contact with epithelial cells it does not cause any specific effects.
Tolerability and safety have been studied in rabbits after a single injection and after use 4 times daily for 4 weeks. No drug-related reactions were observed in any study. Good tolerability of the drug both on the part of the tissues of the eye and the body as a whole was noted.
There was no evidence of teratogenic and mutagenic properties.
Pharmacokinetics
Because of the high molecular weight and cross-linkages in the structure of carbomer it is unlikely to penetrate and accumulate in the eyeball tissue. It is not absorbed into the blood from the lacrimal ducts and the gastrointestinal tract.
Indications
Indications
Diseases accompanied by dry eye syndrome, manifested by compensatory lacrimation, itching, burning, sensation of a foreign body in the eye:
Dry keratoconjunctivitis.
Eye irritation (as a result of exposure to dust, smoke, or when working on a computer – “office eye syndrome”).
Insufficient tear production.
Stevens-Johnson syndrome.
Sjögren’s disease.
Diseases of the eyelids leading to insufficient contact of the cornea with tears.
Rehabilitation therapy after laser and surgical interventions on the cornea.
Pharmacological effect
Pharmacological effect
Oftagel has a good moisturizing effect, contacting the cornea for a long time.
Pharmacodynamics
Polymer chains of carboxyvinyl polymer (polyacrylate) interact with the mucin layer on the corneal epithelium. Non-ionized carboxylic acid residues in the polymer form hydrogen bonds with mucin molecules. The carbomer also contains more ionized regions, which hold water around the molecule through electrostatic forces. In addition to bioadhesion, it increases the viscosity of tears, forming a protective moisturizing film on the surface of the cornea. Thickens the mucin and aqueous layers of the tear film. In contact with epithelial cells, it does not cause any specific effects.
Tolerability and safety were studied in rabbits after a single instillation and after use 4 times a day for 4 weeks. No studies have identified reactions associated with the effects of the drug. The drug was well tolerated both by the tissues of the eye and the body as a whole.
No evidence of teratogenic or mutagenic properties was found.
Pharmacokinetics
Due to the significant molecular weight and the presence of cross-links in the structure of the carbomer, its penetration into the tissue of the eyeball and accumulation in them is unlikely. It is not absorbed into the blood from the lacrimal ducts and gastrointestinal tract.
Special instructions
Special instructions
May cause a temporary decrease in visual acuity. Before driving a car or operating mechanical devices, you should wait until your visual acuity is completely restored.
Between instillations, the bottle should be stored upside down to facilitate the flow of the gel.
Before using the drug, you must wash your hands.
Do not touch the eye or eyelid with the tip of the pipette. After using the drug, you must immediately close the bottle.
Active ingredient
Active ingredient
Carbomer
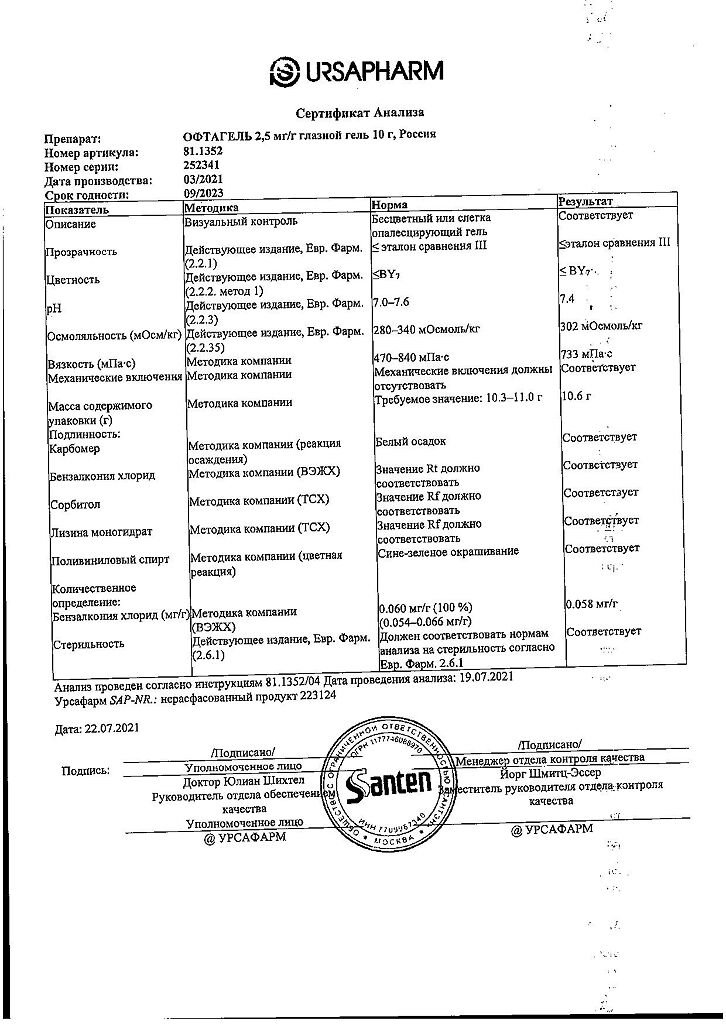
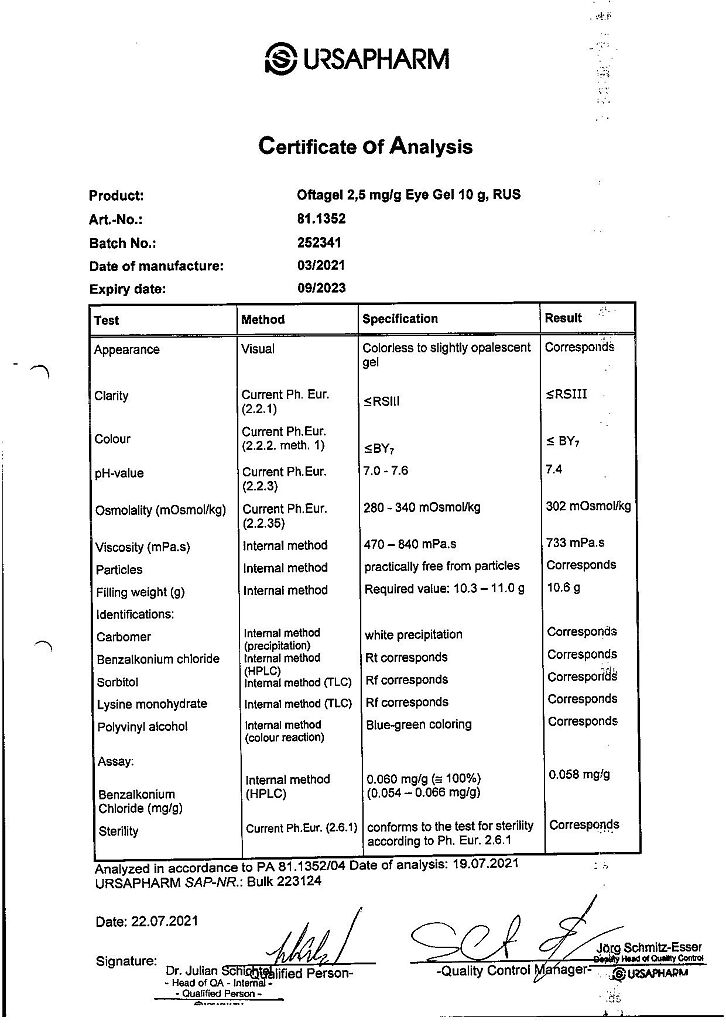
Composition
Composition
Active ingredient:
carbomer 974P 2.5 mg;
Excipients:
benzalkonium chloride;
sorbitol;
lysine monohydrate;
sodium acetate;
polyvinyl alcohol;
water for injections.
Pregnancy
Pregnancy
Use during pregnancy and breastfeeding is possible if the beneficial effect on the mother outweighs the risk to the fetus or child.
The use of the drug during pregnancy and lactation is possible only as prescribed by a doctor.
Contraindications
Contraindications
Hypersensitivity to any component of the drug.
Side Effects
Side Effects
Immediately after instillation, transient blurred vision, a short-term tingling sensation and local eye irritation are possible.
Interaction
Interaction
Prolongs the absorption of other ophthalmic drugs.
If more than one type of eye drop is prescribed, they should be used at least 15 minutes apart, and Oftagel is always instilled last.
Storage conditions
Storage conditions
In a place protected from light, at a temperature of 15–25 °C
Shelf life
Shelf life
2 years
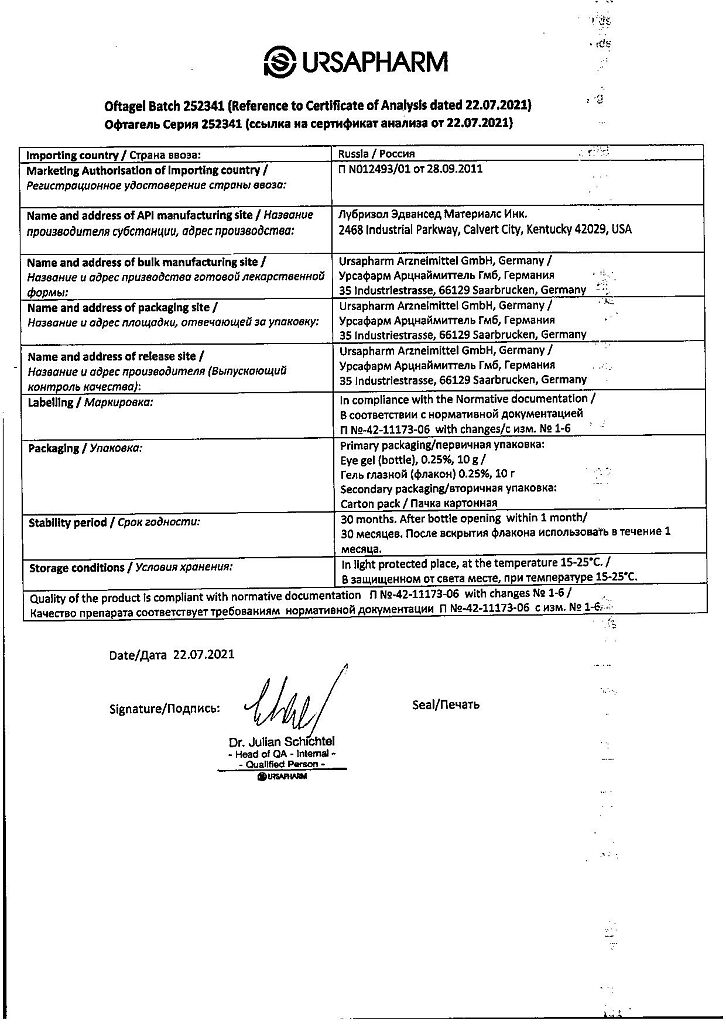
Manufacturer
Manufacturer
Ursafarm Arzneimittel GmbH, Germany
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In a light-protected place at 15-25 °C |
| Manufacturer | NextPharma, Finland |
| Medication form | eye gel |
| Brand | NextPharma |
Related products
Buy Oftagel, eye gel 0.25% 10 g with delivery to USA, UK, Europe and over 120 other countries.