No products in the cart.
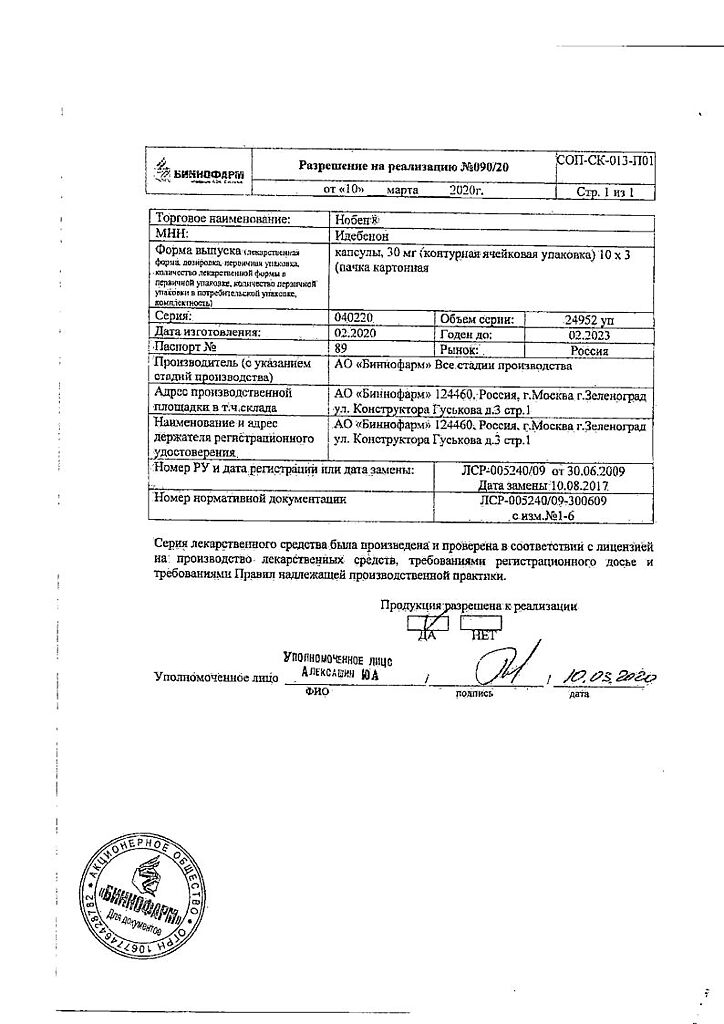
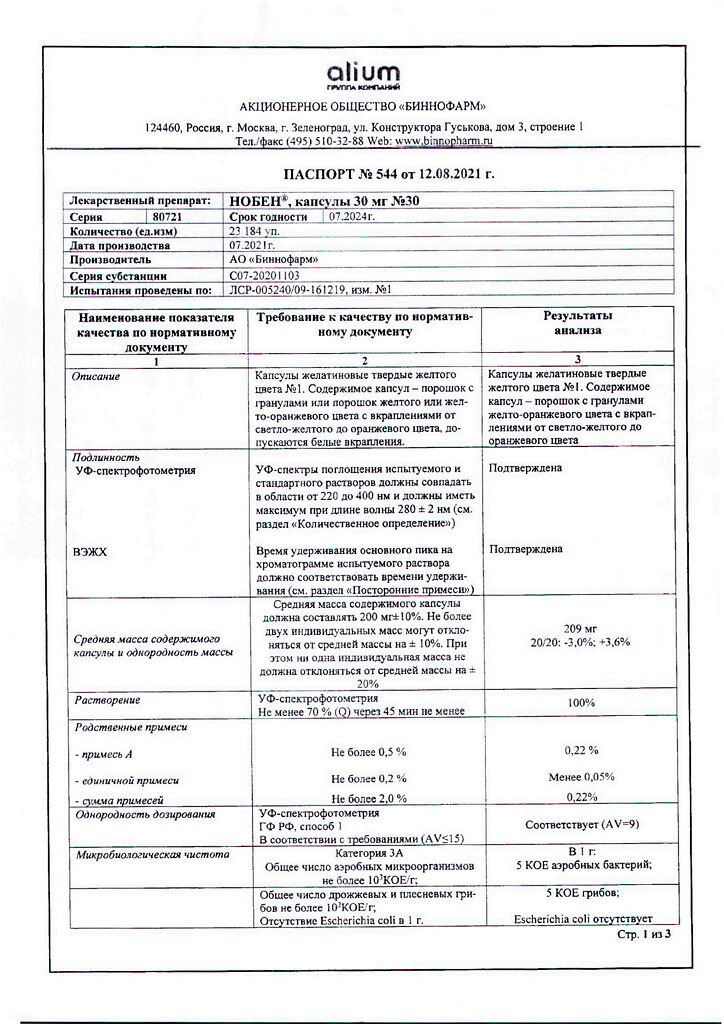
Noben, 30 mg capsules 30 pcs
€24.30 €21.65
Description
Nootropic
Pharmacodynamics
Improves metabolic processes in the brain by activating glucose and ATP synthesis, improving blood supply and oxygenation of brain tissue, promotes lactate excretion. Being an antioxidant, it slows down lipid peroxidation and thereby protects neuronal and mitochondrial membranes from damage. It has nootropic properties with psychostimulatory effect and neuroprotective effect. From the first days of taking the drug, anti-asthenic, psychostimulant and antidepressant effects are manifested. Nootropic and mnemotropic effects are observed after 20-25 days of treatment.
Pharmacokinetics
It is quickly absorbed in the gastrointestinal tract, the peak of concentration in blood is reached after 4 hours. T1/2 is about 18 hours. It easily penetrates through the BBB in significant amounts and is distributed in brain tissues. There is no cumulation even with prolonged use. It is metabolized in the liver and excreted in the urine and faeces.
Indications
Indications
In the treatment of cognitive and behavioral disorders resulting from vascular and degenerative brain pathology.
In the treatment of cognitive and behavioral disorders against the background of cerebrovascular insufficiency and age-related involutional changes in the brain.
In the treatment of cognitive and behavioral disorders against the background of cerebrovascular insufficiency and age-related involutional changes in the brain.
Active ingredient
Active ingredient
Idebenone
Composition
Composition
1 capsule contains:
Active ingredient: idebenone 30 mg.
Excipients: lactose monohydrate, microcrystalline cellulose (type 101), potato starch, povidone (low molecular weight medical polyvinylpyrrolidone or plasdone) (K17), magnesium stearate.
Capsule shell components: titanium dioxide (E171), quinoline yellow dye (E104), sunset yellow dye (E110), gelatin.
Active ingredient: idebenone 30 mg.
Excipients: lactose monohydrate, microcrystalline cellulose (type 101), potato starch, povidone (low molecular weight medical polyvinylpyrrolidone or plasdone) (K17), magnesium stearate.
Capsule shell components: titanium dioxide (E171), quinoline yellow dye (E104), sunset yellow dye (E110), gelatin.
How to take, the dosage
How to take, the dosage
Orally, after meals (last dose no later than 5 p.m.).
30 mg (1 capsule) 2-3 times a day. The course of treatment is determined by the doctor.
30 mg (1 capsule) 2-3 times a day. The course of treatment is determined by the doctor.
Contraindications
Contraindications
Hypersensitivity to idebenone or other components of the drug, chronic renal failure, lactase deficiency, lactose intolerance, glucose-galactose malabsorption, age under 18 years.
With caution
Since idebenone is able to inhibit platelet aggregation in vitro, it is considered that it should be used with caution if there is a history of hemorrhagic stroke or in patients receiving anticoagulants.
Side effects
Side effects
The most common adverse reactions of idebenone (≥ 10%) observed in various clinical studies were: nausea, dyspepsia, diarrhea (mild to moderate, usually not requiring discontinuation of treatment), nasopharyngitis, cough, back pain.
Most clinical studies were conducted under rather specific conditions and higher doses of idebenone were used. For this reason,
Most clinical studies were conducted under rather specific conditions and higher doses of idebenone were used. For this reason,
the frequency of adverse reactions in clinical studies may not reflect their frequency in clinical use. Information on adverse reactions in clinical studies should be considered in order to identify drug-related reactions and their approximate frequency.
Adverse reactions of the drug are systematized for each organ system using the following frequency classification: very common (≥ 1/10), common (≥ 1/100, < 1/10), not known – frequency cannot be estimated from the available data.
Infectious and parasitic diseases: very common: nasopharyngitis; frequency unknown: bronchitis.
Blood and lymphatic system disorders: frequency unknown: leukopenia, thrombocytopenia, neutropenia, agranulocytosis, anaemia.
Immune system disorders: frequency unknown: allergic rhinitis, facial flushing.
Metabolism and nutrition disorders: frequency unknown: increased plasma concentrations of total cholesterol and triglycerides.
Psychiatric disorders: frequency unknown: delirium, hallucinations, agitation, dromomania, anxiety, stupor.
Nervous system disorders: frequency unknown: convulsions, hyperkinesis, dizziness, headache, insomnia, drowsiness.
Respiratory, thoracic and mediastinal disorders:
very common: cough.
Gastrointestinal disorders: common: diarrhoea; Frequency unknown: Dyspepsia, nausea, vomiting, anorexia, abdominal pain.
Hepatobiliary disorders: Frequency unknown: Aspartate aminotransferase increased, hyperbilirubinemia, transient increases in alanine aminotransferase, alkaline phosphatase, lactate dehydrogenase and γ-glutamyl transferase, yellowness of the skin and sclera, hepatitis.
Skin and subcutaneous tissue disorders: Frequency unknown: Rash, pruritus.
Musculoskeletal and connective tissue disorders: Common: Back pain; Frequency unknown: Pain in extremity.
Renal and urinary disorders: Frequency unknown: Plasma urea increased, chromaturia.
General disorders: Frequency unknown: Malaise.
If any of the side effects listed in the instructions get worse, or you notice any other side effects not listed in the instructions, tell your doctor.
Overdose
Overdose
Symptoms: increased severity of dose-dependent side effects.
Treatment: if necessary, prescribe activated charcoal and conduct symptomatic therapy.
Treatment: if necessary, prescribe activated charcoal and conduct symptomatic therapy.
Pregnancy use
Pregnancy use
The safety of the drug in pregnant women has not been established. The drug may be used during pregnancy if the expected benefit to the mother outweighs the possible risk to the fetus.
The drug is contraindicated during breastfeeding. Preclinical studies have shown that idebenone penetrates into breast milk. If it is necessary to use the drug during breastfeeding, breastfeeding should be discontinued.
The drug is contraindicated during breastfeeding. Preclinical studies have shown that idebenone penetrates into breast milk. If it is necessary to use the drug during breastfeeding, breastfeeding should be discontinued.
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | Store in a dry, dark place at a temperature not exceeding +30. Keep out of reach of children. |
| Manufacturer | Binnopharm, Russia |
| Medication form | capsules |
| Brand | Binnopharm |
Related products
Buy Noben, 30 mg capsules 30 pcs with delivery to USA, UK, Europe and over 120 other countries.