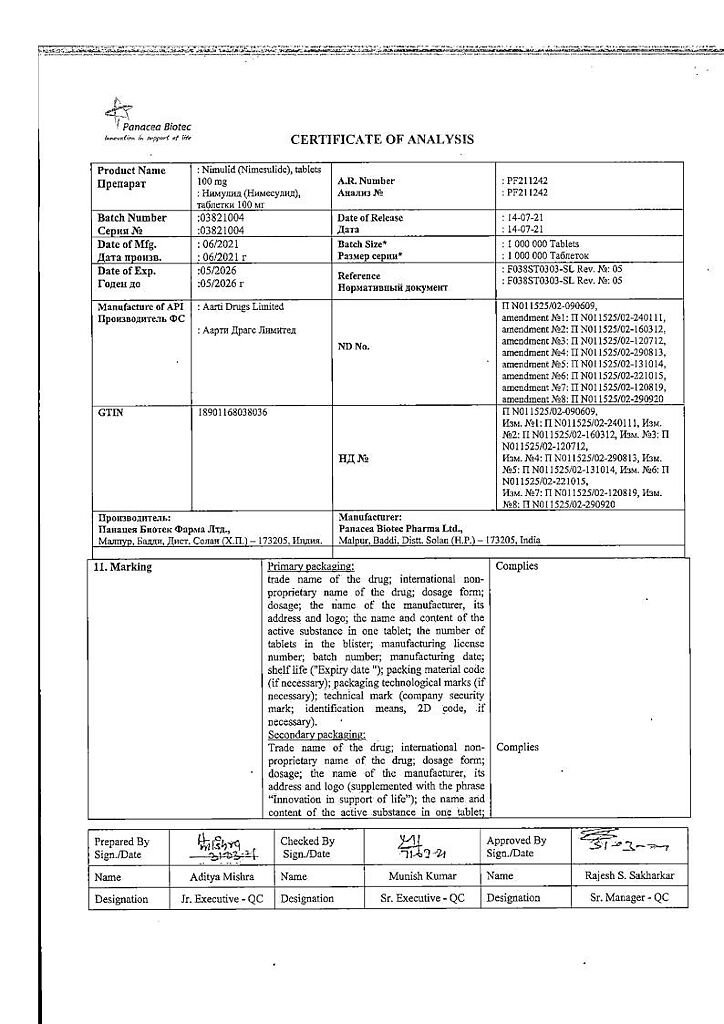
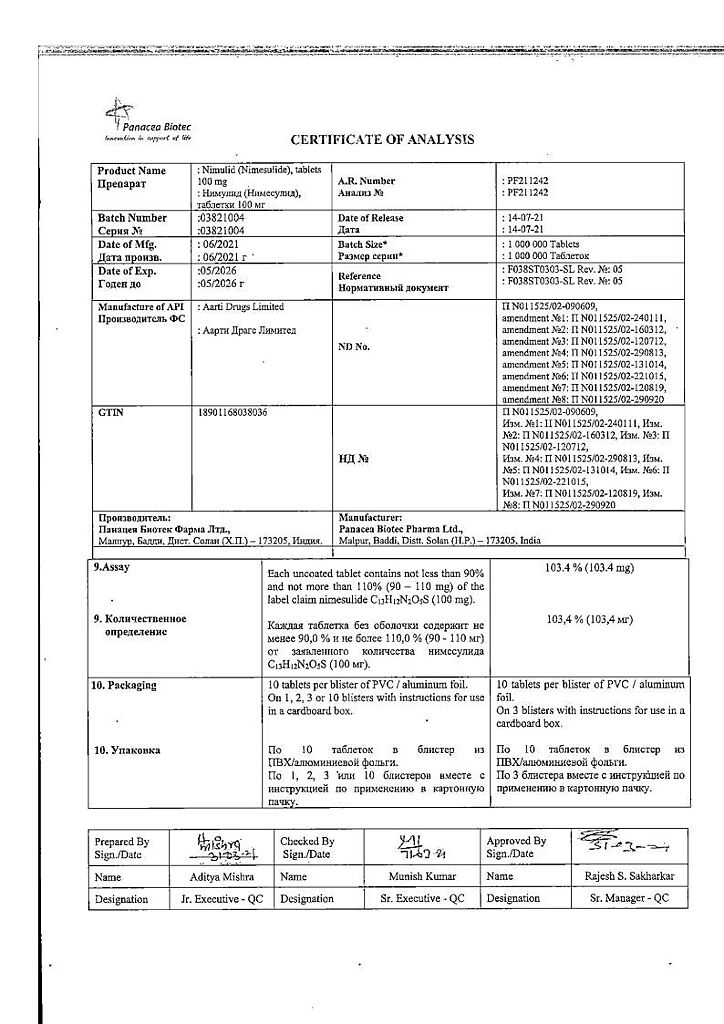
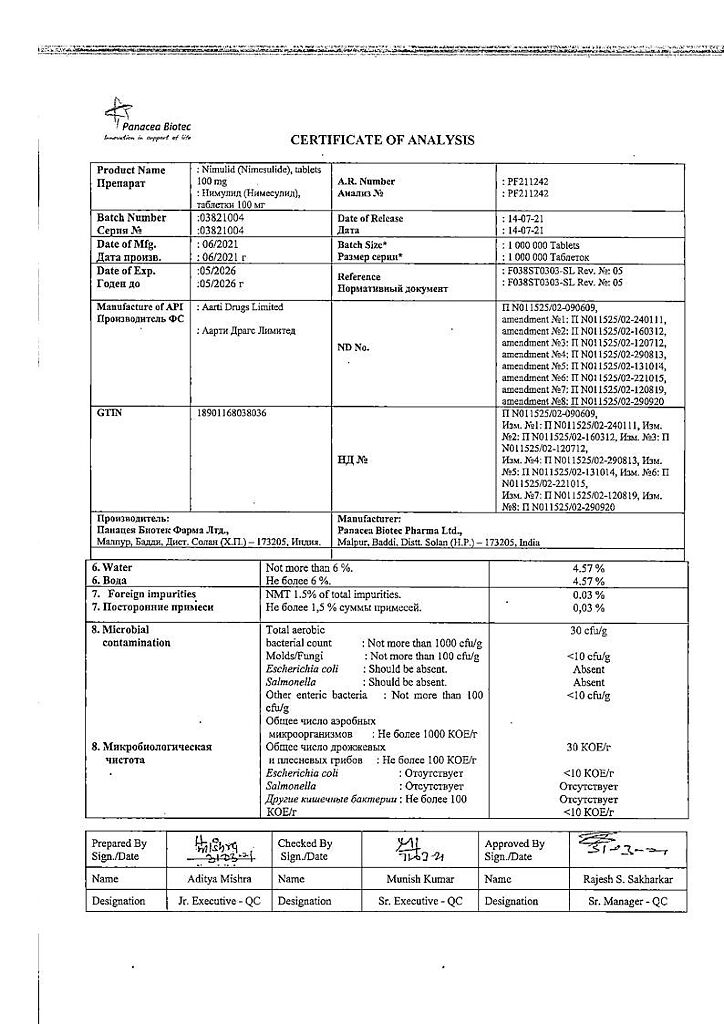
No products in the cart.
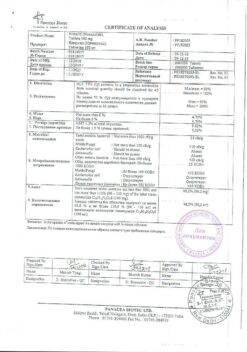
Nimulide, tablets 100 mg 30 pcs
€12.93 €10.78
Description
Nimulide is a non-narcotic anti-inflammatory drug (NSAID) of sulfonanilide class, a selective COX-2 inhibitor. It has anti-inflammatory, analgesic, antipyretic effects.
Nimesulide belongs to NSAIDs, the mechanism of action of which is connected with selective inhibition of COX-2 and influence on several other factors – inhibition of platelet-activating factor, tumor necrosis factor-alpha, inhibition of proteinases and histamine.
With selective inhibition of COX-2 it decreases biosynthesis of PG in inflammation focus, has less pronounced inhibitory effect on COX-1 (causes side effects connected with inhibition of PG synthesis in healthy tissues less frequently).
.
Indications
Indications
– Rheumatoid arthritis,
– articular syndrome during exacerbation of gout,
– psoriatic arthritis,
– ankylosing spondylitis,
– osteochondrosis with radicular syndrome,
– osteoarthritis,
– myalgia of rheumatic and non-rheumatic origin,
– inflammation of ligaments, tendons, bursitis, including post-traumatic inflammation of soft tissues,
– pain syndrome of various origins (including in the postoperative period, with injuries, algodismenorrhea, toothache, headache, arthralgia, lumbar ischialgia).
The drug is intended for symptomatic therapy, reducing pain and inflammation at the time of use, does not affect the progression of the disease.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group
Non-steroidal anti-inflammatory drug (NSAID)
ATX code: M01AX17
Pharmacological action
Nimulide is a non-steroidal, anti-inflammatory drug (NSAID) from the sulfonanilide class. Has anti-inflammatory, analgesic and antipyretic effects.
Nimesulide is a NSAID, the mechanism of action of which is associated with selective inhibition of cyclooxygenase-2 (COX-2) and effects on a number of other factors: suppression of platelet activating factor, tumor necrosis factor alpha, suppression of proteinases and histamine. By selectively suppressing COX-2, it reduces the biosynthesis of prostaglandins at the site of inflammation, has a less pronounced inhibitory effect on COX-1 (less likely to cause side effects associated with inhibition of prostaglandin synthesis in healthy tissues).
Pharmacokinetics
Absorption when taken orally is high (food intake reduces the rate of absorption without affecting its degree). Communication with plasma proteins 95%, with erythrocytes – 2%, with lipoproteins – 1%, with 2 acid alpha 1 -glycoproteins – 1%. Changing the dose does not affect the degree of binding. Cmax – 3.5-6.5 mg/l. Volume of distribution – 0.19-0.35 l/kg. Penetrates into the tissues of the female genital organs, where after a single dose its concentration is about 40% of
plasma concentrations. Penetrates well into the acidic environment of the inflammation site (40%) and synovial fluid (43%). Easily penetrates histohematic barriers. Metabolized in the liver by tissue monooxygenases. The main metabolite, 4-hydroxynimesulide (25%), has similar pharmacological activity, but due to a decrease in molecular size, it is able to quickly diffuse through the hydrophobic COX-2 channel to the active binding site of the methyl group. 4-hydroxynimesulide is a water-soluble compound, the elimination of which does not require glutathione and phase II metabolic conjugation reactions (sulfation, glucuronidation, etc.). T1/2 of nimesulide – about 1.56-4.95 hours, 4-hydroxynimesulide – 2.89-4.78 hours. 4-hydroxynimesulide is excreted by the kidneys (65%) and bile (35%), and undergoes enterohepatic recirculation.
In patients with renal failure (creatinine clearance 1.8-4.8 l/h or 30-80 ml/min), as well as in children and the elderly, the pharmacokinetic profile of nimesulide does not change significantly.
Special instructions
Special instructions
Nimulide should be used with caution in patients who have a history of bleeding, patients with diseases of the upper gastrointestinal tract, or patients receiving anticoagulants. Since Nimulide is partially excreted by the kidneys, its dosage for patients with impaired renal function should be reduced, depending on creatinine clearance.
Given reports of visual disturbances in patients taking other NSAIDs, treatment should be stopped immediately if any visual disturbance occurs and the patient should be examined by an ophthalmologist.
The drug can cause fluid retention in tissues, so patients with high blood pressure and cardiac problems should use Nimulid with extreme caution.
To reduce the risk of developing adverse events from the gastrointestinal tract, the minimum effective dose should be used in the shortest possible short course.
Patients who experience severe side effects: dizziness, drowsiness, blurred vision, must be careful when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Nimesulide
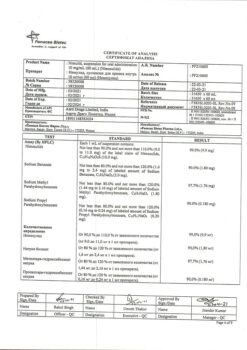
Composition
Composition
Each tablet contains:
Active ingredient: Nimesulide 100.00 mg
Excipients: lactose 151.50 mg, croscarmellose sodium 22.00 mg, colloidal silicon dioxide 11.00 mg, corn starch 37.60 mg, povidone 8.50 mg, docusate sodium 6.80 mg, polysorbate 1.00 mg, hydrochloric acid*, purified water*, magnesium stearate 1.60 mg (* – removed during production).
Pregnancy
Pregnancy
Contraindicated during pregnancy and lactation.
Contraindications
Contraindications
– erosive and ulcerative changes in the mucous membrane of the stomach and duodenum;
– inflammatory bowel diseases in the acute phase;
– active gastrointestinal bleeding;
– severe impairment of liver and/or kidney function, severe renal failure (creatinine clearance less than 30 ml/min), progressive kidney disease;
– severe liver failure or active liver disease;
– pregnancy and lactation;
– children’s age up to 12 years;
– hypersensitivity to nimesulide and drug components;
– severe heart failure;
– severe blood clotting disorders;
– confirmed hyperkalemia;
– period after coronary artery bypass grafting;
– anamnestic data on an attack of bronchial obstruction, rhinitis, urticaria after taking acetylsalicylic acid or other NSAIDs (complete or incomplete acetylsalicylic acid intolerance syndrome – rhinosinusitis, urticaria, nasal polyps, asthma).
With caution
Coronary heart disease, cerebrovascular disease, congestive heart failure, dyslipidemia/hyperlipidemia, diabetes mellitus, peripheral arterial disease, smoking, creatinine clearance less than 60 ml/min.
Anamnestic data on the development of ulcerative lesions of the gastrointestinal tract, the presence of Helicobacter pylori infection, old age, long-term use of NSAIDs, frequent alcohol consumption, severe somatic diseases, concomitant therapy with the following drugs:
– Anticoagulants (eg warfarin)
– antiplatelet agents (for example, acetylsalicylic acid, clopidogrel)
– oral glucocorticosteroids (for example, prednisolone)
– selective serotonin reuptake inhibitors (for example, citalopram, fluoxetine, paroxetine, sertraline)
Side Effects
Side Effects
The frequency is classified according to the headings, depending on the occurrence of the case: very often (>10), often (<10-<100), infrequently (<100-<1000), rarely (<1000-<10000), very rarely (<10000).Gastrointestinal tract: often – diarrhea, nausea, vomiting; infrequently – constipation, flatulence, gastritis; very rarely – abdominal pain, stomatitis, tarry stools, gastrointestinal bleeding, ulcer and/or perforation of the stomach or duodenum.
Central nervous system: infrequently – dizziness; rarely – a feeling of fear, nervousness, nightmares; very rarely – headache, drowsiness, encephalopathy (Reye’s syndrome).
Respiratory organs: infrequently – shortness of breath; very rarely – bronchospasm; possible exacerbation of bronchial asthma.
Cardiovascular system: infrequently – arterial hypertension; rarely – tachycardia, hemorrhages, “hot flashes”.
Sense organs: rarely – blurred vision.
Skin and mucous membranes: infrequently – itching, rash, increased sweating; rarely: erythema, dermatitis.
Liver and biliary system: often – increased activity of “liver” transaminases; very rarely – hepatitis, fulminant hepatitis, jaundice, cholestasis.
Kidneys and urinary system: infrequently – edema; rarely – dysuria, hematuria, urinary retention, hyperkalemia; very rarely – renal failure, oliguria, interstitial nephritis.
Hematopoietic organs: rarely – anemia, eosinophilia; very rarely – thrombocytopenia, pancytopenia, purpura, prolonged bleeding time.
Allergic reactions: rarely – hypersensitivity reactions; very rarely – anaphylactoid reactions, urticaria, angioedema, facial swelling, exudative erythema multiforme, incl. Stevens-Johnson syndrome, toxic epidermal necrolysis (Lyell’s syndrome)
General reactions: rarely – general weakness; very rarely – hypothermia.
If you experience other side effects not mentioned above or if your health worsens, please contact your doctor immediately.
Interaction
Interaction
The effect of medications that reduce blood clotting is enhanced when used simultaneously with nimesulide.
Nimesulide may reduce the effect of furosemide. Reduces the therapeutic effect of antihypertensive drugs. Nimesulide increases the occurrence of side effects while taking methotrexate.
Plasma lithium levels increase when lithium and nimesulide are taken simultaneously.
Nimesulide may enhance the nephrotoxic effect of cyclosporine on the kidneys.
Use with glucocorticosteroids and serotonin reuptake inhibitors increases the risk of gastrointestinal bleeding.
Overdose
Overdose
Symptoms: apathy, drowsiness, nausea, vomiting. Gastrointestinal bleeding, arterial hypertension, acute renal failure, and respiratory depression may occur.
Treatment: symptomatic treatment of the patient is required; there is no specific antidote. If an overdose occurs within the last 4 hours, it is necessary to induce vomiting, take activated carbon (60-100 g per adult), and osmotic laxatives. Forced diuresis and hemodialysis are ineffective due to the high binding of the drug to proteins.
Storage conditions
Storage conditions
In a place protected from light at a temperature of 15 – 25 ° C.
Keep out of the reach of children.
Shelf life
Shelf life
5 years.
Do not use the drug after the expiration date indicated on the package.
Manufacturer
Manufacturer
Panacea Biotech, India
Additional information
| Shelf life | 5 years |
|---|---|
| Conditions of storage | In a dry, light-protected place at 15-25 °C |
| Manufacturer | Panacea Biotec, India |
| Medication form | pills |
| Brand | Panacea Biotec |
Other forms…
Related products
Buy Nimulide, tablets 100 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.