No products in the cart.
Movasin, 10 mg/ml 1.5 ml 5 pcs.
€12.57 €10.47
Description
NSAIDs. It has analgesic, anti-inflammatory and antipyretic effects. The mechanism of action is related to inhibition of prostaglandin synthesis as a result of selective inhibition of enzymatic activity of cyclooxygenase-2 (COX-2), which participates in biosynthesis of prostaglandins in inflammation area. When administered in high doses, prolonged use and individual characteristics of the body selectivity against COX-2 decreases.
It suppresses prostaglandin synthesis in the area of inflammation to a greater extent than in the gastric mucosa or kidneys, which is associated with relatively selective inhibition of COX-2. Less often causes erosive and ulcerative diseases of the gastrointestinal tract. To a lesser extent, meloxicam acts on cyclooxygenase-1 (COX-1), which is involved in the synthesis of prostaglandins that protect the gastrointestinal mucosa and are involved in the regulation of blood flow in the kidneys.
Pharmacokinetics
Intake
After oral administration of the drug meloxicam is well absorbed from the gastrointestinal tract, the absolute bioavailability is 89%. Concomitant use with food does not change absorption. Magnitude of meloxicam concentration when administered orally in doses of 7.5 and 15 mg is proportional to the dose.
The relative bioavailability is almost 100% after administration in m/m. After an oral dose of 5 mg, Cmax is 1.62 mcg/ml and is reached within approximately 60 min.
Meloxicam exhibits linear pharmacokinetics at doses of 7.5-15 mg when administered by injection.
Distribution
Css are achieved within 3-5 days of regular administration. With long-term use of the drug (more than 1 year), concentrations are similar to those observed after first achieving steady state pharmacokinetics. Binding to plasma proteins (especially albumin) is more than 99%.
The range of differences between maximal and basal concentrations of the drug after its administration once daily is relatively small and amounts to 0.4-1 mcg/ml with 7.5 mg dose and 0.8-2 mcg/ml with 15 mg dose (Cmin and Cmax values are given, respectively).
Meloxicam penetrates the histohematic barriers, the concentration in synovial fluid reaches 50% of the maximum concentration of the drug in plasma.
The Vd is low and is 11 liters. Interindividual differences are 30-40%.
Metabolism
Meloxicam is almost completely metabolized in the liver to form four pharmacologically inactive metabolites. The main metabolite 5′-carboxymeloxicam (60% of the dose value) is formed by oxidation of the intermediate metabolite 5′-hydroxymethylmeloxicam, which is also excreted, but to a lesser extent (9% of the dose value). In vitro studies have shown that CYP2C9 isoenzyme plays an important role in this metabolic transformation, CYP3A4 isoenzyme has additional importance. Peroxidase, the activity of which probably varies individually, is involved in the formation of the other two metabolites (constituting, respectively, 16% and 4% of the value of the drug dose).
Extracted equally through the intestine and kidneys, mainly as metabolites. Less than 5% of the daily dose is excreted unchanged in the intestine, the drug is detected only in trace amounts in the urine. T1/2 of meloxicam after oral administration is 15-20 hours, when administered by injection – 20 hours. Plasma clearance averages 8 ml/min.
Pharmacokinetics in special clinical cases
The drug clearance is decreased in elderly persons.
Hepatic or renal insufficiency of moderate severity have no significant effect on the pharmacokinetics of meloxicam.
Indications
Indications
Symptomatic therapy:
– osteoarthritis;
– rheumatoid arthritis;
– ankylosing spondylitis (ankylosing spondylitis).
Pharmacological effect
Pharmacological effect
NSAIDs. It has analgesic, anti-inflammatory and antipyretic effects. The mechanism of action is associated with inhibition of prostaglandin synthesis as a result of selective suppression of the enzymatic activity of cyclooxygenase-2 (COX-2), involved in the biosynthesis of prostaglandins in the area of inflammation. When prescribed in high doses, long-term use and individual characteristics of the body, selectivity for COX-2 decreases.
Suppresses the synthesis of prostaglandins in the area of inflammation to a greater extent than in the gastric mucosa or kidneys, which is associated with relatively selective inhibition of COX-2. Less commonly causes erosive and ulcerative diseases of the gastrointestinal tract. To a lesser extent, meloxicam acts on cyclooxygenase-1 (COX-1), which is involved in the synthesis of prostaglandins that protect the gastrointestinal mucosa and are involved in the regulation of blood flow in the kidneys.
Pharmacokinetics
Suction
After taking the drug orally, meloxicam is well absorbed from the gastrointestinal tract, absolute bioavailability is 89%. Concomitant use with food does not alter absorption. The concentration of meloxicam when taking the drug orally in doses of 7.5 and 15 mg is proportional to the dose.
After intramuscular administration, the relative bioavailability is almost 100%. After intramuscular administration of the drug at a dose of 5 mg, Cmax is 1.62 mcg/ml and is achieved within approximately 60 minutes.
Meloxicam exhibits linear pharmacokinetics in doses of 7.5-15 mg when administered intramuscularly.
Distribution
Css are achieved within 3-5 days of regular use. With long-term use of the drug (more than 1 year), concentrations are similar to those observed after the first achievement of a steady state of pharmacokinetics. Binding to plasma proteins (especially albumin) is more than 99%.
The range of differences between the maximum and basal concentrations of the drug after taking it once a day is relatively small and is 0.4-1 μg/ml when using a dose of 7.5 mg, and 0.8-2 μg/ml when using a dose of 15 mg (Cmin and Cmax values are given, respectively).
Meloxicam penetrates histohematic barriers, the concentration in synovial fluid reaches 50% of the maximum concentration of the drug in plasma.
Vd is low and is 11 l. Interindividual differences are 30-40%.
Metabolism
Meloxicam is almost completely metabolized in the liver to form four pharmacologically inactive metabolites. The main metabolite 5′-carboxymeloxicam (60% of the dose) is formed by oxidation of the intermediate metabolite 5′-hydroxymethylmeloxicam, which is also excreted, but to a lesser extent (9% of the dose). In vitro studies have shown that the CYP2C9 isoenzyme plays an important role in this metabolic transformation, and the CYP3A4 isoenzyme is of additional importance. Peroxidase is involved in the formation of the other two metabolites (constituting, respectively, 16% and 4% of the drug dose), the activity of which probably varies individually.
Removal
It is excreted equally through the intestines and kidneys, mainly in the form of metabolites. Less than 5% of the daily dose is excreted unchanged through the intestines; the drug is found unchanged in urine only in trace amounts. T1/2 of meloxicam after oral administration is 15-20 hours, with intramuscular administration – 20 hours. Plasma clearance averages 8 ml/min.
Pharmacokinetics in special clinical situations
In elderly people, drug clearance is reduced.
Hepatic or renal failure of moderate severity does not have a significant effect on the pharmacokinetics of meloxicam.
Special instructions
Special instructions
Caution should be exercised when using the drug in patients with a history of gastric and duodenal ulcers, as well as in patients on anticoagulant therapy. Such patients have an increased risk of erosive and ulcerative diseases of the gastrointestinal tract.
Caution should be exercised and renal function monitored when using the drug in elderly patients, in patients with chronic heart failure with clinical manifestations, in patients with liver cirrhosis, as well as in patients with hypovolemia as a result of surgical interventions.
In patients with renal failure, if CC is more than 30 ml/min, no dosage adjustment is required.
In patients on dialysis, the dosage of the drug should not exceed 7.5 mg/day.
Patients taking diuretics and meloxicam simultaneously should take sufficient fluids.
If allergic reactions occur during treatment (itching, skin rash, urticaria, photosensitivity), you must consult a doctor to decide whether to stop taking the drug.
Meloxicam, like other NSAIDs, can mask the symptoms of infectious diseases.
The use of meloxicam, like other drugs that block prostaglandin synthesis, can affect fertility and is therefore not recommended for women planning pregnancy.
Active ingredient
Active ingredient
Meloxicam
Composition
Composition
Active ingredient:
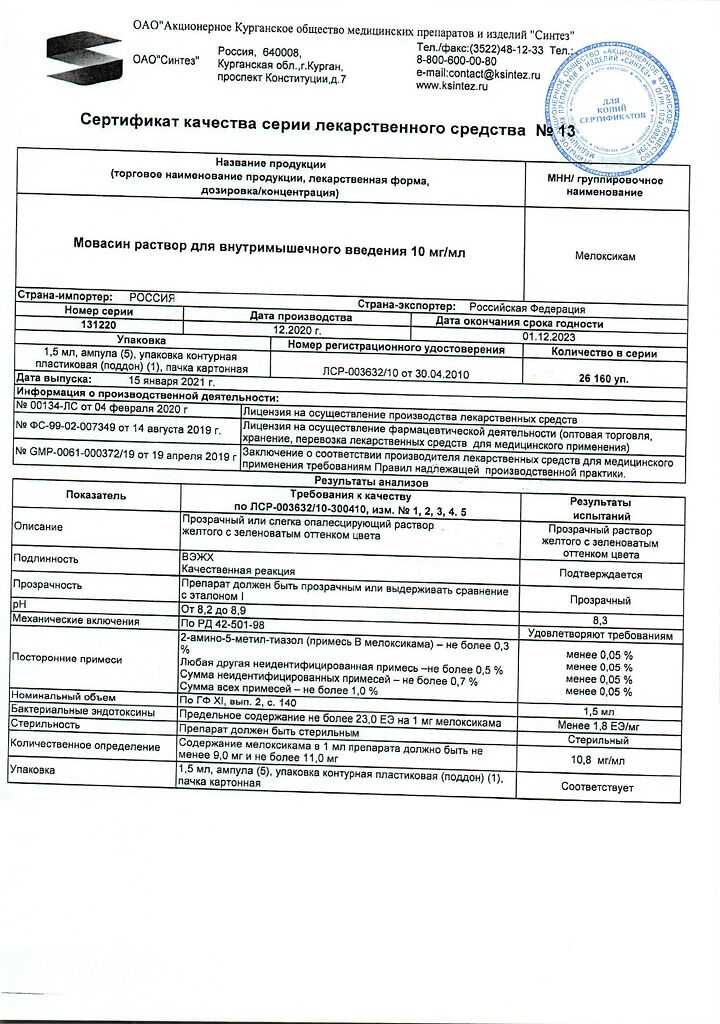
meloxicam – 10 mg,
Excipients:
sodium chloride,
glycine,
glycofurfural (tetraglycol),
poloxamer 188,
meglumine (meglumine),
sodium hydroxide,
water for injections.
Contraindications
Contraindications
— condition after coronary artery bypass surgery;
– decompensated heart failure;
– complete or incomplete combination of bronchial asthma, recurrent polyposis of the nasal mucosa and paranasal sinuses and intolerance to acetylsalicylic acid and other NSAIDs (including a history);
– erosive and ulcerative changes in the mucous membrane of the stomach or duodenum, active gastrointestinal bleeding;
– inflammatory bowel diseases (ulcerative colitis, Crohn’s disease);
– cerebrovascular bleeding or other bleeding;
– severe liver failure or active liver disease;
– chronic renal failure in patients not undergoing dialysis (creatinine clearance less than 30 ml/min), progressive kidney diseases (including confirmed hyperkalemia);
– children under 12 years of age (for tablets);
– children under 18 years of age (for solution for intramuscular administration);
– pregnancy;
– lactation (breastfeeding);
– hypersensitivity to meloxicam and other components of the drug.
The drug in tablet form contains lactose, so patients with rare hereditary diseases such as galactose intolerance, lactase deficiency or glucose-galactose malabsorption should not take the drug.
With caution
– IHD;
– cerebrovascular diseases;
– compensated heart failure;
— dyslipidemia/hyperlipidemia;
– diabetes mellitus;
— diseases of peripheral arteries;
– smoking;
— CC 30-60 ml/min;
— anamnestic data on the development of ulcerative lesions of the gastrointestinal tract;
— presence of Helicobacter pylori infection;
– old age;
– long-term use of NSAIDs;
– frequent alcohol consumption;
– severe somatic diseases;
– concomitant therapy with the following drugs: anticoagulants (for example, warfarin); antiplatelet agents (for example, acetylsalicylic acid, clopidogrel); oral corticosteroids (for example, prednisolone); selective serotonin reuptake inhibitors (eg, citalopram, fluoxetine, paroxetine, sertraline).
To reduce the risk of developing adverse events from the gastrointestinal tract, the minimum effective dose should be used for the shortest possible short course.
Side Effects
Side Effects
From the digestive system: more than 1% – dyspepsia, incl. nausea, vomiting, abdominal pain, constipation, flatulence, diarrhea; 0.1-1% – transient increase in the activity of “liver” transaminases, hyperbilirubinemia, belching, esophagitis, gastroduodenal ulcer, bleeding from the gastrointestinal tract (including hidden), stomatitis; less than 0.1% – gastrointestinal perforation, colitis, hepatitis, gastritis.
From the hematopoietic system: more than 1% – anemia; 0.1-1% – change in blood formula, incl. leukopenia, thrombocytopenia.
From the skin: more than 1% – itching, skin rash; 0.1-1% – urticaria; less than 0.1% – photosensitivity, bullous rashes, erythema multiforme, incl. Stevens-Johnson syndrome, toxic epidermal necrolysis.
From the respiratory system: less than 0.1% – bronchospasm.
From the central nervous system (CNS): more than 1% – dizziness, headache; 0.1-1% – vertigo, tinnitus, drowsiness; less than 0.1% – confusion, disorientation, emotional lability.
From the cardiovascular system (CVS): more than 1% – peripheral edema; 0.1-1% – increased blood pressure (BP), palpitations, “flushes” of blood to the skin of the face.
From the urinary system: 0.1-1% – hypercreatininemia and/or increased urea in the blood serum; less than 0.1% – acute renal failure; no connection with meloxicam has been established – interstitial nephritis, albuminuria, hematuria.
From the senses: less than 0.1% – conjunctivitis, visual impairment, incl. blurred vision.
Allergic reactions: less than 0.1% – angioedema, anaphylactic/anaphylactoid reactions.
Local reactions: more than 1% – swelling at the injection site; less than 1% – pain at the injection site
Interaction
Interaction
When taken simultaneously with other NSAIDs (as well as acetylsalicylic acid), the risk of erosive and ulcerative lesions and bleeding from the gastrointestinal tract increases.
When used simultaneously with antihypertensive drugs, the effectiveness of the latter may be reduced.
When used simultaneously with lithium preparations, the development of accumulation of lithium and an increase in its toxic effect are possible (monitoring the concentration of lithium in the blood is recommended).
When used simultaneously with methotrexate, the latter’s side effect on the hematopoietic system increases (the risk of anemia and leukopenia; periodic monitoring of a general blood test is indicated).
When used simultaneously with diuretics and cyclosporine, the risk of developing renal failure increases.
When used simultaneously with intrauterine contraceptives, the effectiveness of the latter may decrease.
When used simultaneously with anticoagulants (heparin, ticlopidine, warfarin), antiplatelet agents (acetylsalicylic acid, clopidogrel), as well as with fibrinolytic drugs (streptokinase, fibrinolysin), the risk of bleeding increases (periodic monitoring of blood clotting parameters is necessary).
When used simultaneously with selective serotonin reuptake inhibitors, the risk of gastrointestinal bleeding increases.
Overdose
Overdose
Symptoms: impaired consciousness, nausea, vomiting, epigastric pain, gastrointestinal bleeding, acute renal failure, liver failure, respiratory arrest, asystole.
Treatment: there is no specific antidote; symptomatic therapy. Forced diuresis, alkalization of urine, hemodialysis are ineffective due to the high binding of the drug to blood proteins.
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 30 ºС.
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Sintez, Russia
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature not exceeding 30 ºC. |
| Manufacturer | Sintez OAO, Russia |
| Medication form | solution |
| Brand | Sintez OAO |
Related products
Buy Movasin, 10 mg/ml 1.5 ml 5 pcs. with delivery to USA, UK, Europe and over 120 other countries.