No products in the cart.
Midantan, 100 mg 100 pcs
€6.99 €5.86
Description
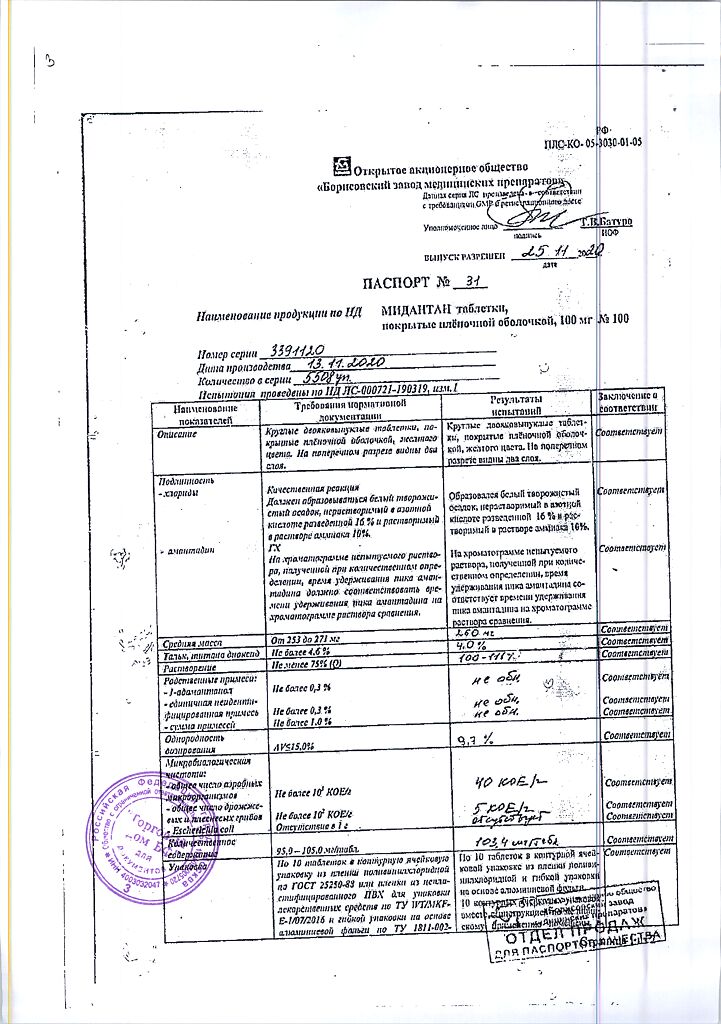
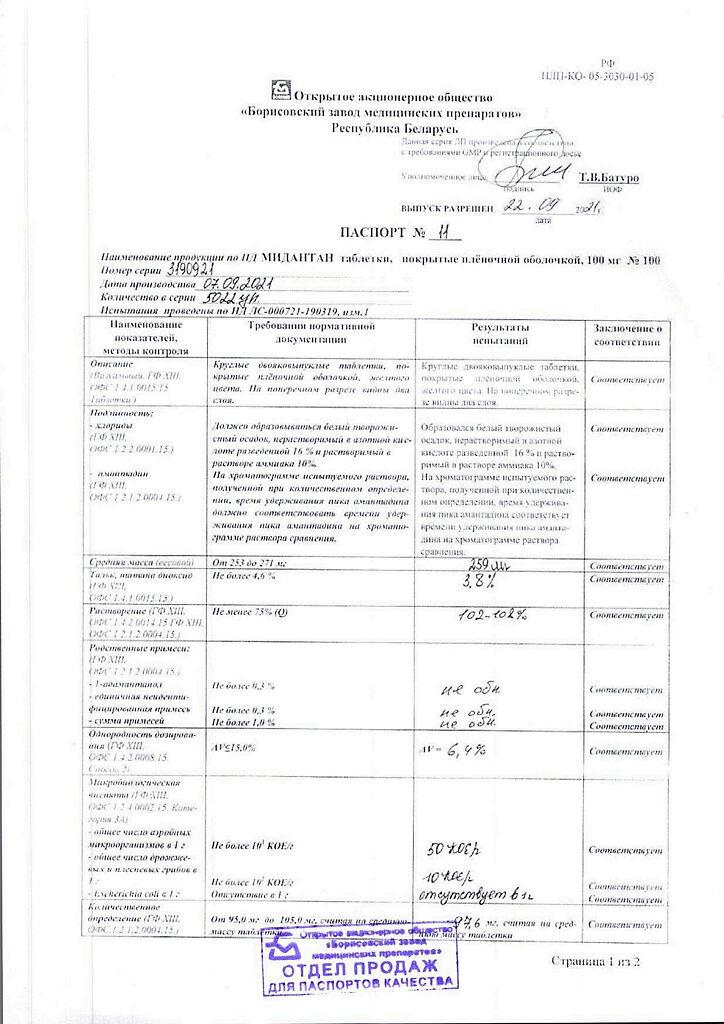
Midantan is an antiparkinsonian drug, a tricyclic symmetric adamantamine.
Blocks glutamate NMDA-receptors (including in the substantia nigra), thus reducing the excessive stimulating effect of cortical glutamate neurons on the neostriatum. developing against the background of insufficient dopamine release. By reducing the inflow of ionized Ca2+ into neurons, it reduces the possibility of their destruction.
The greater effect is on stiffness (rigidity and bradykyesia).
Pharmacokinetics
After oral administration, it is well absorbed in the GI tract. Cmax in plasma – in 5 hours; T1/2 of amantadine sulfate – 12-13 hours, of amantadine hydrochloride – 30 hours. It is excreted unchanged by kidneys.
Indications
Indications
Parkinson’s disease, parkinsonism syndrome.
Pharmacological effect
Pharmacological effect
Midantan is an antiparkinsonian drug, a tricyclic symmetric adamantamine.
Blocks glutamate NMDA receptors (including in the substantia nigra), thereby reducing the excessive stimulating effect of cortical glutamate neurons on the neostriatum. developing against the background of insufficient release of dopamine. By reducing the flow of ionized Ca2+ into neurons, it reduces the possibility of their destruction.
It has a greater effect on stiffness (rigidity and bradycyesia).
Pharmacokinetics
After oral administration it is well absorbed from the gastrointestinal tract. Cmax in blood plasma after 5 hours; T1/2 amantadine sulfate – 12-13 hours, amantadine hydrochloride – 30 hours. Excreted unchanged by the kidneys.
Special instructions
Special instructions
Information about the effectiveness in the treatment of extrapyramidal disorders during treatment with antipsychotic drugs (drug-induced parkinsonism) is contradictory. Therapy should not be stopped suddenly, because a sharp exacerbation of the disease is possible. The use of ethanol while taking the drug is contraindicated.
During the treatment period, it is necessary to refrain from driving vehicles and engaging in potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Amantadine
Composition
Composition
1 tablet contains:
active ingredient:
amantadine hydrochloride – 100 mg;
excipients:
lactose monohydrate, starch 1500 (partially pregelatinized corn starch),
croscarmellose sodium,
talc,
stearic acid,
potato starch,
opadry II (contains: polyvinyl alcohol, partially hydrolyzed, macrogol 3350, talc, titanium dioxide E 171, aluminum varnish based on yellow quinoline E 104, yellow iron oxide E 172).
Pregnancy
Pregnancy
Contraindicated during pregnancy (first trimester) and lactation. Take with caution in the 2nd and 3rd trimesters of pregnancy.
Contraindications
Contraindications
Liver failure, chronic renal failure, psychosis (including history), thyrotoxicosis, epilepsy, angle-closure glaucoma, prostatic hyperplasia, arterial hypotension, chronic heart failure stage II–III, state of agitation, predelirium, delirious psychosis, pregnancy planning, pregnancy, lactation, simultaneous use of triamterene and hydrochlorothiazide, a history of gastric or duodenal ulcer, hypersensitivity to amantadine or other components of the drug, children under 18 years of age.
Side Effects
Side Effects
From the nervous system: motor or mental agitation, convulsions, headache, dizziness, irritability, insomnia, tremor, mental disorders accompanied by visual hallucinations.
From the cardiovascular system: heart failure, tachycardia, orthostatic hypotension, arrhythmogenic effect.
From the digestive system: dry mouth, nausea, anorexia, dyspepsia.
From the urinary system: acute urinary retention in patients with prostatic hyperplasia, polyuria, nocturia.
Other: dermatosis, the appearance of a bluish coloration of the skin of the upper and lower extremities, decreased visual acuity.
Interaction
Interaction
Medicines that stimulate the central nervous system and ethanol increase the risk of side effects. Enhances the effect of levodopa and psychostimulants. Compatible with central anticholinergics and other antiparkinsonian drugs.
Storage conditions
Storage conditions
In a dry place, at a temperature not exceeding 25 °C
Shelf life
Shelf life
5 years
Manufacturer
Manufacturer
Borisov Medical Preparations Plant, Belarus
Additional information
| Shelf life | 5 years |
|---|---|
| Conditions of storage | In a dry place, at a temperature not exceeding 25 °C |
| Manufacturer | Borisov Medical Preparations Plant, Belarus |
| Medication form | pills |
| Brand | Borisov Medical Preparations Plant |
Related products
Buy Midantan, 100 mg 100 pcs with delivery to USA, UK, Europe and over 120 other countries.