Subtotal: €41.81
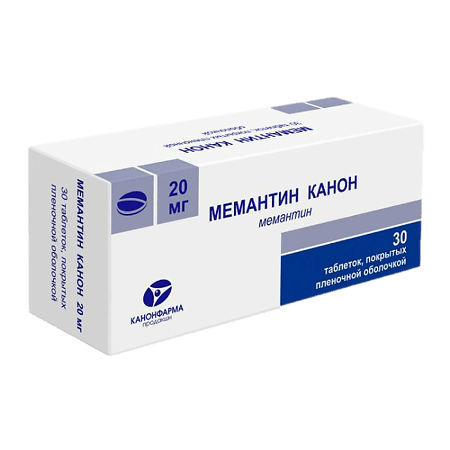
Memantine Canon, 20 mg 30 pcs
€44.93 €37.44
Out of stock
(E-mail when Stock is available)
Concussion and other brain injuries, Alcoholism, Stroke sequelae, Alzheimer’s disease, Attention and memory disorders, Acquired dementiaModerate to severe Alzheimer’s dementia.
Indications
Moderate to severe dementia of the Alzheimer’s type.
Pharmacological effect
Pharmacodynamics
Special instructions
It is recommended to use with caution in patients with epilepsy, a history of seizures, or in patients with a predisposition to epilepsy.
Active ingredient
Memantine
Composition
Dosage 20 mg
Contraindications
Hypersensitivity to the active substance or any of the components of the drug;
Severe liver failure (class C according to the Child-Pugh classification);
Congenital galactose intolerance, lactose deficiency or glucose/galactose malabsorption syndrome;
Pregnancy;
Breastfeeding period;
Children under 18 years of age (efficacy and safety have not been established).
With caution:
Epilepsy, history of seizures;
History of myocardial infarction;
Heart failure (III-IV classes according to the NYHA classification);
Uncontrolled arterial hypertension;
Simultaneous use of NMDA receptor antagonists (amantadine, ketamine, dextromethorphan);
Factors that increase urine pH (sudden change of diet (change from meat to vegetarian), heavy intake of alkaline gastric buffers, renal tubular acidosis or severe urinary tract infections caused by Proteus spp.);
Kidney failure;
Liver failure.
Side Effects
The following are undesirable reactions obtained both from studies and from spontaneous reports.
WHO classification of the incidence of adverse reactions: very often (≥1/10); often (≥1/100, <1/10); uncommon (≥1/1000, <1/100); rare (≥1/10000, <1/1000); very rare (< 1/10000),
frequency unknown – based on available data, it is not possible to determine the frequency of occurrence.
Interaction
Levodopa, dopamine receptor agonists and m-anticholinergic drugs. With the simultaneous use of memantine with levodopa drugs, dopamine receptor antagonists, and m-anticholinergic blockers, the effect of the latter may be enhanced, as with simultaneous use with other NMDA receptor antagonists.
Overdose
During ongoing clinical trials and post-marketing studies of the drug Memantine Canon, a limited amount of information on overdoses was obtained.
Symptoms: With sufficiently large overdoses (200 mg once or more than 100 mg per day for 3 days), the following symptoms were identified: weakness, fatigue, diarrhea or absence of symptoms. With an overdose of less than 140 mg or with an unknown overdose, the following adverse effects from the nervous system were identified: confusion, drowsiness, dizziness, agitation, aggression, hallucinations and gait disturbance and from the gastrointestinal tract: vomiting and diarrhea.
In the most serious cases of overdose, the patient survived after taking more than 2000 mg of memantine with adverse effects on the nervous system (coma for 10 days, diplopia, agitation). The patient received symptomatic therapy and plasmapheresis and recovered without any consequences.
Another reported case of serious overdose is 400 mg once. The patient recovered without consequences. Reactions from the nervous system were noted: anxiety, psychosis, visual hallucinations, stupor, drowsiness, unconsciousness.
Treatment: in case of overdose, symptomatic treatment is necessary. There is no specific antidote for memantine intoxication. Carry out standard procedures for evacuating the drug by washing, using activated carbon, forced diuresis, and urine alkalizing agents.
If symptoms of over-irritation of the central nervous system appear, symptomatic therapy should be selected very carefully.
Manufacturer
Kanonpharma production CJSC, Russia
| Manufacturer | Kanonfarma Production ZAO, Russia |
|---|---|
| Medication form | pills |
| Brand | Kanonfarma Production ZAO |
Related products
Buy Memantine Canon, 20 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.