No products in the cart.
Maltofer, 10 mg/ml syrup 150 ml
€11124.00 €9.27
Description
Maltofer contains iron in the form of a polymaltose complex of iron (III) hydroxide. < br>
This macromolecular complex is stable and does not release iron in the form of free ions in the gastrointestinal tract. The structure of Maltofer® is similar to the natural iron compound – ferritin. < br>
Thanks to this similarity, iron (III) is delivered from the intestine into the blood by active transport. Absorbed iron is bound to ferritin and stored in the body, mainly in the liver. < br>
It is then incorporated into hemoglobin in the bone marrow. < br>
The iron in the polymaltose complex of iron (III) hydroxide does not have pro-oxidant properties, unlike simple iron salts. < br>
There is a correlation between the severity of iron deficiency and the level of iron absorption (the greater the severity of iron deficiency, the better the absorption). < br>
The most active absorption process occurs in the duodenum and small intestine.
The drug Maltofer® does not cause staining of dental enamel.
< br>
Indications
Indications
Latent iron deficiency and clinically pronounced iron deficiency (iron deficiency anemia);
prevention of iron deficiency in women during pregnancy, breastfeeding, during the childbearing period, in children, incl. in adolescence, in adults (for example, vegetarians and the elderly).
Pharmacological effect
Pharmacological effect
Maltofer contains iron in the form of a polymaltose complex of iron (III) hydroxide.
This macromolecular complex is stable and does not release iron in the form of free ions in the gastrointestinal tract. The structure of Maltofer® is similar to the natural iron compound ferritin.
Due to this similarity, iron (III) moves from the intestine into the blood through active transport. Absorbed iron binds to ferritin and is stored in the body, mainly in the liver.
It is then incorporated into hemoglobin in the bone marrow.
Iron, which is part of the polymaltose complex of iron (III) hydroxide, does not have pro-oxidant properties, unlike simple iron salts.
There is a correlation between the severity of iron deficiency and the level of its absorption (the greater the severity of iron deficiency, the better the absorption).
The most active absorption process occurs in the duodenum and small intestine.
Maltofer® does not stain tooth enamel.
Special instructions
Special instructions
When prescribing the drug to patients with diabetes, it should be taken into account that 1 ml of drops for oral administration contains 0.01 XE, 1 ml of syrup – 0.04 XE, 1 chewable tablet – 0.04 XE.
Maltofer® does not stain tooth enamel.
Active ingredient
Active ingredient
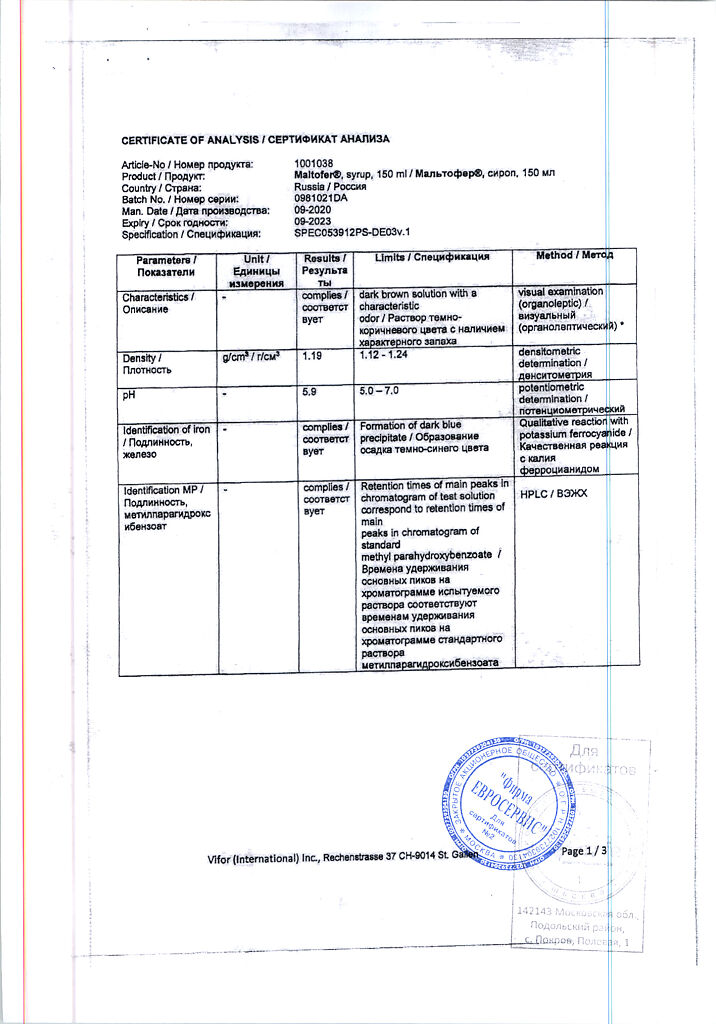
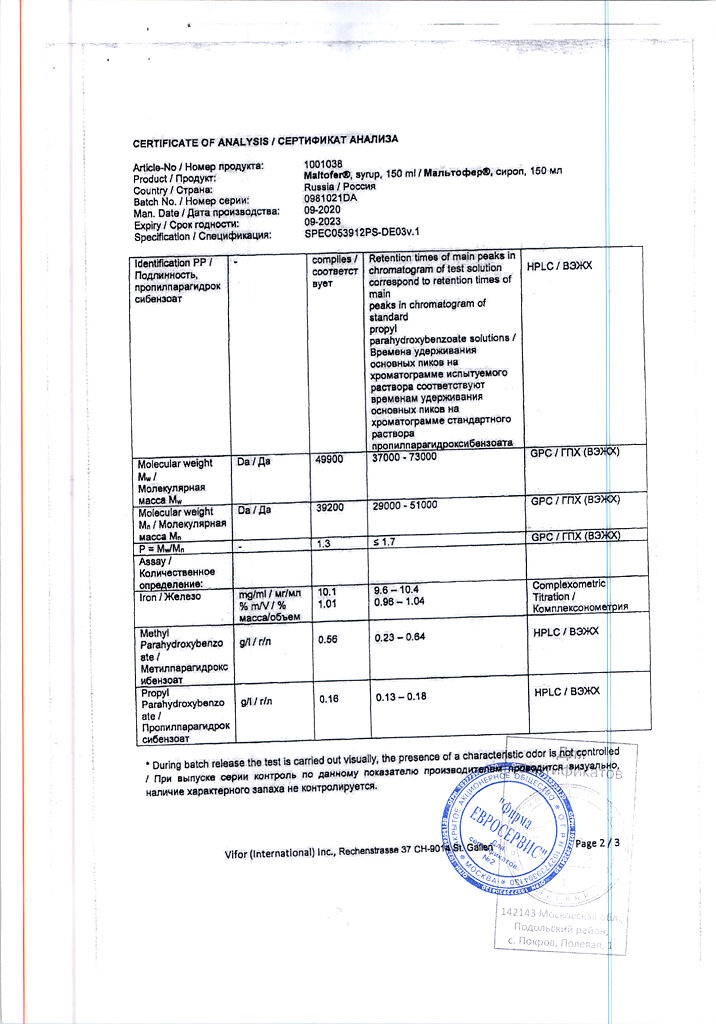
Iron III hydroxide polymaltosate
Composition
Composition
Active ingredient:
iron in the form of polymaltose complex of iron (III) hydroxide 10 mg;
Excipients:
methyl parahydroxybenzoate;
propyl parahydroxybenzoate;
sodium hydroxide;
sorbitol solution 70%;
ethanol 96% (3.25 mg);
water;
sucrose;
cream flavoring;
Pregnancy
Pregnancy
In controlled studies in pregnant women after the first trimester of pregnancy, no undesirable effects of the drug on the mother and fetus were observed.
There is no data on the undesirable effect of the drug on the fetus in the first trimester of pregnancy.
Contraindications
Contraindications
iron overload (eg hemosiderosis and hemochromatosis);
impaired iron utilization (lead anemia, sideroachrestic anemia);
non-iron deficiency anemia (hemolytic or megaloblastic, caused by a lack of vitamin B12).
Side Effects
Side Effects
From the gastrointestinal tract: a feeling of fullness, pressure in the epigastric region, nausea, constipation or diarrhea; Possible dark coloration of stool due to the release of unabsorbed iron, which has no clinical significance.
Interaction
Interaction
No interactions with other drugs have been identified.
Overdose
Overdose
To date, neither intoxication nor signs of iron overload have been reported in cases of drug overdose.
Storage conditions
Storage conditions
In a place protected from light, at a temperature not exceeding 25 °C
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Vifor S.A., Switzerland
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a light-protected place, at a temperature not exceeding 25 °C |
| Manufacturer | Vifor S.A., Switzerland |
| Medication form | syrup |
| Brand | Vifor S.A. |
Other forms…
Related products
Buy Maltofer, 10 mg/ml syrup 150 ml with delivery to USA, UK, Europe and over 120 other countries.