No products in the cart.
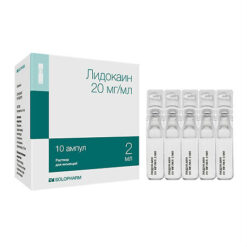
Lidocaine, 20 mg/ml 2 ml 5 pcs
€0.96 €0.87
Description
Pharmacotherapeutic group
Local anesthetic agent
ATCode
N01BB
Pharmacodynamics: Lidocaine is a short-acting local anesthetic of the amide type. Its mechanism of action is based on reduction of permeability of neuronal membrane for sodium ions. As a result, the rate of depolarization decreases and the excitation threshold increases, resulting in reversible local numbness. Lidocaine is used to achieve conductive anesthesia in various parts of the body and to control arrhythmias. It has a rapid onset of action (about one minute after intravenous injection and fifteen minutes after intramuscular injection) and quickly spreads to surrounding tissues. The action lasts 10-20 minutes and about 60-90 minutes after intravenous and intramuscular injection, respectively. Pharmacokinetics:
Absorption
Lidocaine is rapidly absorbed from the gastrointestinal tract but due to the “primary passage” effect through the liver, only small amounts reach the systemic bloodstream. Systemic absorption of lidocaine is determined by the site of administration and its pharmacological profile. The maximum concentration in the blood is reached after intercostal blockade, then (in order of decreasing concentration) after injection in the lumbar epidural space brachial plexus and subcutaneous tissues. The main factor determining the rate of absorption and concentration in the blood is the total dose administered, regardless of the site of administration. There is a linear relationship between the amount of lidocaine injected and the resulting maximum blood concentration of the anesthetic.
Distribution
Lidocaine binds to plasma proteins including α1-acid glycoprotein (ACG) and albumin. The degree of binding is variable at approximately 66%. Plasma concentrations of AKG in newborns are low, so they have relatively high levels of free bioactive fraction of lidocaine. Lidocaine penetrates through the blood-brain and placental barriers probably by passive diffusion. Metabolism
Lidocaine is metabolized in the liver about 90% of the administered dose undergoes N-dealkylation to form monoethylglycincylidide (MEGX) and glycincylidide (GX) both contributing to the therapeutic and toxic effects of lidocaine. The pharmacological and toxic effects of MEGX and GX are comparable to those of lidocaine but are weaker. GX has a longer half-life than lidocaine (about 10 hours) and may cumulate with repeated administration. Metabolites resulting from subsequent metabolism are excreted in the urine. The content of unchanged lidocaine in the urine is less than 10%.
The terminal elimination half-life of lidocaine after intravenous bolus administration in healthy adult volunteers is 1-2 hours. The terminal half-life of GX is approximately 10 hours MEGX is 2 hours.
Particular patient groups
Due to rapid metabolism, the pharmacokinetics of lidocaine may be affected by conditions that impair hepatic function. In patients with hepatic dysfunction the elimination half-life of lidocaine may be 2 or more times longer. Impaired renal function does not affect the pharmacokinetics of lidocaine but may lead to cumulation of its metabolites.
In newborns there are low concentrations of ACG so binding to plasma proteins may be reduced. Because of the potentially high concentration of the free fraction, the use of lidocaine in neonates is not recommended.
Indications
Indications
Local and regional anesthesia, conduction anesthesia for major and minor interventions.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group
Local anesthetic
ATX code
N01BB
Pharmacodynamics:
Lidocaine is a short-acting amide-type local anesthetic. Its mechanism of action is based on a decrease in the permeability of the neuron membrane to sodium ions. As a result, the rate of depolarization decreases and the excitation threshold increases, leading to reversible local numbness. Lidocaine is used to achieve conduction anesthesia in various parts of the body and control arrhythmias. It has a rapid onset of action (about one minute after intravenous administration and fifteen minutes after intramuscular administration) and quickly spreads into surrounding tissues. The action lasts 10-20 minutes and about 60-90 minutes after intravenous and intramuscular administration, respectively.
Pharmacokinetics:
Absorption
Lidocaine is rapidly absorbed from the gastrointestinal tract; however, due to the first pass effect through the liver, only a small amount reaches the systemic circulation. Systemic absorption of lidocaine is determined by the site of dose administration and its pharmacological profile. The maximum concentration in the blood is achieved after intercostal blockade, then (in order of decreasing concentration) after injection into the lumbar epidural space, the brachial plexus and subcutaneous tissue. The main factor determining the rate of absorption and concentration in the blood is the total dose administered, regardless of the injection site. There is a linear relationship between the amount of lidocaine administered and the resulting maximum concentration of anesthetic in the blood.
Distribution
Lidocaine binds to plasma proteins including α1-acid glycoprotein (ACG) and albumin. The degree of binding is variable, being approximately 66%. The plasma concentration of ACH in newborns is low; therefore, they have a relatively high content of the free biologically active fraction of lidocaine. Lidocaine penetrates the blood-brain and placental barriers, probably through passive diffusion. Metabolism
Lidocaine is metabolized in the liver, approximately 90% of the administered dose undergoes N-dealkylation to form monoethylglycine xylidide (MEGX) and glycine xylidide (GX), both of which contribute to the therapeutic and toxic effects of lidocaine. The pharmacological and toxic effects of MEGX and GX are comparable to those of lidocaine but are less pronounced. GX has a longer half-life than lidocaine (about 10 hours) and can accumulate with repeated administration. Metabolites formed as a result of subsequent metabolism are excreted in the urine; the content of unchanged lidocaine in the urine does not exceed 10%.
Removal
The terminal half-life of lidocaine after intravenous bolus administration to healthy adult volunteers is 1-2 hours. The terminal half-life of GX is approximately 10 hours; MEGX is 2 hours.
Special patient groups
Due to rapid metabolism, the pharmacokinetics of lidocaine may be influenced by conditions that impair liver function. In patients with hepatic dysfunction, the half-life of lidocaine may increase by 2 or more times. Impaired renal function does not affect the pharmacokinetics of lidocaine but can lead to the accumulation of its metabolites.
In newborns, there is a low concentration of AKG, so the connection with plasma proteins may be reduced. Due to the potentially high concentration of the free fraction, the use of lidocaine in newborns is not recommended.
Special instructions
Special instructions
Regional and local anesthesia should be carried out by experienced specialists in an appropriately equipped room with the availability of equipment and drugs necessary for cardiac monitoring and resuscitation, ready for immediate use. Personnel performing anesthesia must be qualified and trained in anesthesia techniques and must be familiar with the diagnosis and treatment of systemic toxic reactions, adverse events and reactions and other complications.
It should be used with caution in patients with myasthenia gravis, epilepsy, chronic heart failure, bradycardia and respiratory depression, as well as in combination with drugs that interact with lidocaine and lead to an increase in its bioavailability, potentiation of effects (for example, phenytoin) or prolongation of elimination (for example, in hepatic or end-stage renal failure in which lidocaine metabolites can accumulate). Patients receiving class III antiarrhythmic drugs (eg amiodarone) should be closely monitored and ECG monitoring since the effect on the heart may be potentiated.
There have been post-marketing reports of chondrolysis in patients receiving prolonged intra-articular infusion of local anesthetics after surgery. In most cases, chondrolysis was observed in the shoulder joint. Due to the many contributing factors and the inconsistency in the scientific literature regarding the mechanism by which the effect is realized, a cause-and-effect relationship has not been identified. Long-term intra-articular infusion is not an approved indication for the use of lidocaine. Intramuscular administration of lidocaine may increase the activity of creatine phosphokinase, which may complicate the diagnosis of acute myocardial infarction.
Lidocaine has been shown to cause porphyria in animals and its use should be avoided in persons with porphyria.
When injected into inflamed or infected tissues, the effect of lidocaine may be reduced.
Before starting intravenous lidocaine, it is necessary to eliminate hypokalemia, hypoxia and acid-base imbalance.
Some local anesthetic procedures may cause serious adverse reactions, regardless of the local anesthetic used.
Conduction anesthesia of the spinal nerves can lead to depression of the cardiovascular system, especially against the background of hypovolemia; therefore, caution should be exercised when performing idural anesthesia in patients with cardiovascular disorders.
Epidural anesthesia can lead to hypotension and bradycardia. The risk can be reduced by pre-administration of crystalloid or colloid solutions. Arterial hypertension must be stopped immediately. In some cases, paracervical blockade during pregnancy can lead to bradycardia or tachycardia in the fetus, which requires careful monitoring of the fetal heart rate (see section “Use during pregnancy and breastfeeding”).
Administration to the head and neck area may result in inadvertent arterial entry leading to cerebral symptoms, even at low doses. Retrobulbar administration may, in rare cases, result in entry into the subarachnoid space of the skull resulting in serious/severe reactions including cardiovascular insufficiency, seizure apnea, and temporary blindness. Retro- and peribulbar administration of local anesthetics carries a low risk of persistent oculomotor dysfunction. The main causes include injury and/or local toxicity to muscles and/or nerves.
The severity of such reactions depends on the degree of injury, the concentration of the local anesthetic and the duration of its exposure in the tissues. In this regard, any local anesthetic must be used in the lowest effective concentration and dose. Lidocaine injection solution is not recommended for use in newborns. The optimal serum concentration of lidocaine to avoid toxicity such as seizures and arrhythmias in newborns has not been established. Intravascular administration should be avoided unless directly indicated.
Use with caution:
– in patients with coagulopathy. Therapy with anticoagulants (eg heparin), NSAIDs or plasma expanders increases the tendency to bleeding. Accidental damage to blood vessels can lead to severe bleeding. If necessary, the bleeding time, activated partial thromboplastin time (aPTT) and platelet count should be checked;
– patients with complete or incomplete block of intracardiac conduction, since local anesthetics can inhibit AV conduction;
– Patients with seizure disorders should be carefully monitored for central nervous system symptoms. Low doses of lidocaine may also increase seizure activity. In patients with Melkersson-Rosenthal syndrome, allergic and toxic reactions from the nervous system in response to the administration of local anesthetics may develop more often;
– third trimester of pregnancy.
Lidocaine injection solution 20 mg/ml is not approved for intrathecal administration (subarachnoid anesthesia).
Impact on the ability to drive vehicles. Wed and fur.:
After administration of local anesthetics, a transient decrease in sensitivity and/or motor block may occur. Until these effects resolve, patients are not recommended to drive vehicles or operate machinery.
Active ingredient
Active ingredient
Lidocaine
Composition
Composition
Per 1 ml:
active substance:
lidocaine hydrochloride monohydrate (in terms of lidocaine hydrochloride) – 20 mg;
excipients:
sodium chloride – 6 mg,
sodium hydroxide 1 M solution – up to pH 5.0-7.0,
water for injection – up to 1 ml.
Pregnancy
Pregnancy
Fertility
There are no data on the effect of lidocaine on human fertility.
Pregnancy
Lidocaine can be used during pregnancy and breastfeeding. It is necessary to strictly adhere to the prescribed dosage regimen. If there are complications or a history of bleeding, epidural anesthesia with lidocaine in obstetrics is contraindicated.
Lidocaine has been used in a large number of pregnant women and women of childbearing age. No reproductive disorders were registered i.e. There was no increase in the incidence of developmental defects.
Due to the potential for high concentrations of local anesthetics to be achieved in the fetus after a paracervical block, it may develop adverse reactions such as fetal bradycardia. In this regard, lidocaine in a concentration exceeding 1% is not used in obstetrics. No harmful effects on the fetus were found in animal studies.
Breast-feeding
Lidocaine passes into breast milk in small amounts; its oral bioavailability is very low. Thus, the expected amount obtained through breast milk is very small and therefore the potential harm to the baby is very low. The decision about the possibility of using lidocaine during breastfeeding is made by the doctor.
Contraindications
Contraindications
– Hypersensitivity to the components of the drug and to amide-type anesthetics;
– 3rd degree atrioventricular (AV) block;
– hypovolemia.
With caution:
The administration of lidocaine should be carried out with caution in patients with myasthenia gravis, epilepsy, chronic heart failure, bradycardia and respiratory depression, coagulopathy, complete or incomplete blockade of intracardiac conduction, convulsive disorders, Melkersson-Rosenthal syndrome, porphyria, as well as in the third trimester of pregnancy.
Side Effects
Side Effects
Adverse reactions are described according to MedDRA system organ classes. Frequency is defined as follows:
Like other local anesthetics, adverse reactions to lidocaine are rare and are usually due to increased plasma concentrations due to accidental intravascular overdose or rapid absorption from areas of abundant blood supply or due to idiosyncratic hypersensitivity or reduced patient tolerance. Systemic toxicity reactions mainly occur in the central nervous and/or cardiovascular systems (see also section “Overdose”).
Immune system disorders
Hypersensitivity reactions (allergic or anaphylactoid reactions, anaphylactic shock) – see also skin and subcutaneous tissue disorders. The lidocaine skin allergy test is considered unreliable.
Nervous system and mental disorders
Neurological signs of systemic toxicity include dizziness, nervousness, tremors, paresthesia around the mouth, numbness of the tongue, drowsiness, convulsions, coma. Reactions from the nervous system can manifest themselves as either excitation or depression. Signs of central nervous system stimulation may be short-lived or not occur at all, as a result of which the first manifestations of toxicity may be confusion and drowsiness, followed by coma and respiratory failure. Neurological complications of spinal anesthesia include transient neurological symptoms such as pain in the lower back, buttocks and legs. These symptoms usually develop within 24 hours after anesthesia and resolve within a few days. After spinal anesthesia with lidocaine and similar agents, isolated cases of arachnoiditis and cauda equina syndrome with persistent paresthesia, intestinal and urinary tract dysfunction, or paralysis of the lower extremities have been described. Most cases are caused by hyperbaric concentrations of lidocaine or prolonged spinal infusion.
Visual disorders
Signs of lidocaine toxicity may include blurred vision, diplopia, and transient amaurosis.
Bilateral amaurosis can also result from accidental insertion into the optic nerve bed during ophthalmic procedures. After retro- and peribulbar anesthesia, ocular inflammation and diplopia have been reported (see section “Special Instructions”).
Disorders of the hearing organ and labyrinth
Tinnitus hyperacusis.
Cardiovascular disorders
Cardiovascular reactions are manifested by arterial hypotension, bradycardia, myocardial depression (negative inotropic effect), arrhythmias, possible cardiac arrest or circulatory failure.
Disorders of the respiratory system of the chest and mediastinum Dyspnea bronchospasm respiratory depression respiratory arrest.
Gastrointestinal disorders
Nausea and vomiting.
Skin and subcutaneous tissue disorders
Rash urticaria angioedema swelling of the face.
Interaction
Interaction
The toxicity of lidocaine increases when it is used simultaneously with cimetidine and propranolol due to an increase in the concentration of lidocaine, which requires a reduction in the dose of lidocaine. Both drugs reduce hepatic blood flow. In addition, cimetidine inhibits microsomal activity. Ranitidine slightly reduces the clearance of lidocaine, which leads to an increase in its concentration. Increased serum concentrations of lidocaine can also be caused by antiviral agents (for example, amprenavir atazanavir darunavir lopinavir).
Hypokalemia caused by diuretics can reduce the effect of lidocaine when used simultaneously (see section “Special Instructions”).
Lidocaine should be used with caution in patients receiving other local anesthetics or agents structurally similar to amide-type local anesthetics (for example, antiarrhythmics such as mexiletine tocainide) since systemic toxic effects are additive. Separate studies of drug interactions between lidocaine and class III antiarrhythmics (eg amiodarone) have not been conducted; however, caution is recommended. Patients concomitantly receiving antipsychotics that prolong or are capable of prolonging the QT interval (for example, pimozide sertindole olanzaine quetiapine zotepine), prenylamine epinephrine (with occasional intravenous administration) or 5-HT3-serotonin receptor antagonists (for example, tropisetron dolasetron) may increase the risk of ventricular arrhythmias.
Concomitant use of quinupristin/dalfopristin may increase lidocaine concentrations and thus increase the risk of ventricular arrhythmias; their simultaneous use should be avoided.
Patients receiving concomitant muscle relaxants (eg suxamethonium) may have an increased risk of enhanced and prolonged neuromuscular blockade. Cardiovascular failure has been reported following the use of bupivaca in patients receiving verapamil and timolol; lidocaine is similar in structure to bupivacaine.
Dopamine and 5-hydroxytryptamines lower the seizure threshold to lidocaine. Opioids appear to have a convulsant effect, supported by evidence that lidocaine lowers the seizure threshold to fentanyl in humans.
A combination of opioids and antiemetics, sometimes used for sedation in children, may lower the seizure threshold to lidocaine and increase its CNS depressant effect.
The use of epinephrine with lidocaine may reduce systemic absorption, but with accidental intravenous administration the risk of ventricular tachycardia and ventricular fibrillation increases sharply.
The simultaneous use of other antiarrhythmics, beta-blockers and slow calcium channel blockers may further reduce AV conduction through the ventricles and contractility.
The simultaneous use of vasoconstrictors increases the duration of action of lidocaine.
Concomitant use of lidocaine and ergot alkaloids (eg ergotamine) can cause severe hypotension.
Caution must be exercised when using sedatives as they may affect the effect of local anesthetics on the central nervous system.
Caution should be exercised with long-term use of antiepileptic drugs (phenytoin), barbiturates and other inhibitors of microsomal liver enzymes as this may lead to decreased effectiveness and, as a result, an increased need for lidocaine.
On the other hand, intravenous administration of phenytoin can enhance the inhibitory effect of lidocaine on the heart.
The analgesic effect of local anesthetics may be enhanced by opioids and clonidip.
Ethyl alcohol, especially with prolonged abuse, can reduce the effect of local anesthetics.
Lidocaine is incompatible with amphotericin B methohexitone and nitroglycerin. Mixing lidocaine with other medications is not recommended.
Overdose
Overdose
Symptoms
Toxicity from the central nervous system is manifested by symptoms of increasing severity. First, paresthesia around the mouth, numbness of the tongue, dizziness, hyperacusis and tinnitus may develop. Visual disturbances and muscle tremors or muscle twitching indicate more serious toxicity and precede generalized seizures. These signs should not be confused with neurotic behavior. Loss of consciousness and grand mal seizures may then occur, lasting from a few seconds to several minutes. Convulsions lead to a rapid increase in hypoxia and hypercapnia caused by increased muscle activity and respiratory failure. In severe cases, apnea may develop. Acidosis enhances the toxic effects of local anesthetics.
In severe cases, disorders of the cardiovascular system occur. At high systemic concentrations, arterial hypotension, bradycardia, arrhythmia and cardiac arrest may develop, which can be fatal.
Resolution of an overdose occurs due to the redistribution of the local anesthetic from the central nervous system and its metabolism; it can occur quite quickly (unless a very large dose of the drug has been administered).
Treatment
If signs of overdose occur, the administration of the anesthetic should be stopped immediately.
Seizures, CNS depression and cardiotoxicity require medical intervention. The main goals of therapy are to maintain oxygenation and stop seizures
maintaining blood circulation and relieving acidosis (if it develops). In appropriate cases, it is necessary to ensure patency of the airways and administer oxygen, as well as establish auxiliary ventilation (mask or using an Ambu bag). Blood circulation is maintained by infusion of plasma or infusion solutions. If long-term maintenance of blood circulation is necessary, the possibility of administering vasopressors should be considered; however, they increase the risk of central nervous system excitation. Control of seizures can be achieved by intravenous administration of diazepam (01 mg/kg) or sodium thiopentane (1-3 mg/kg), but it should be borne in mind that anticonvulsants may also depress respiration and circulation. Prolonged convulsions may interfere with the patient’s ventilation and oxygenation, and early endotracheal intubation should be considered. If the heart stops, standard cardiopulmonary resuscitation begins.
The effectiveness of dialysis in the treatment of acute lidocaine overdose is very low.
Storage conditions
Storage conditions
Store in a place protected from light at a temperature not exceeding 25 °C. Keep out of the reach of children.
Shelf life
Shelf life
2 years.
Do not use after the expiration date indicated on the package.
Manufacturer
Manufacturer
Belmedpreparaty, Belarus
Additional information
| Shelf life | 2 years. Do not use after the expiration date printed on the package. |
|---|---|
| Conditions of storage | Store in a light-protected place at a temperature not exceeding 25 °С. Keep out of reach of children. |
| Manufacturer | Belmedpreparaty, Belarus |
| Medication form | solution for injection |
| Brand | Belmedpreparaty |
Other forms…
Related products
Buy Lidocaine, 20 mg/ml 2 ml 5 pcs with delivery to USA, UK, Europe and over 120 other countries.