No products in the cart.
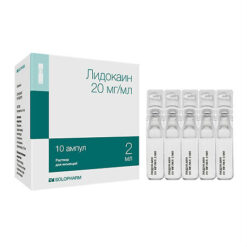
Lidocaine, 20 mg/ml 2 ml 10 pcs
€1.48 €1.35
Description
It is used in:
- terminal (superficial) mucosal anesthesia (in urology, bronchoscopy, etc.);
- infiltration anesthesia during surgical operations;
- for peripheral nerve anesthesia (in dentistry, limb surgery, etc.
- for blockade of nerve plexuses, (brachial lumbar, etc.);
- for epidural and spinal (spinal) anesthesia,
- local anesthesia in ophthalmology.
Lidocaine hydrochloride is used as an antiarrhythmic agent in the treatment of mono- and polytopic extrasystoles and ventricular tachycardia (including those which occur during general anesthesia, during cardiac surgery, in overdose of cardiac glycosides and others.), to stop ventricular fibrillation in the acute phase of myocardial infarction, as well as in ventricular defibrillation as a component of pharmacotherapy. In addition, it is used for the prevention of ventricular extrasystoles and tachycardia of any origin.
Indications
Indications
Used for:
terminal (superficial) anesthesia of mucous membranes (in urology, during bronchoscopy, etc.);
infiltration anesthesia during surgical operations;
for anesthesia of peripheral nerves (in dentistry, limb surgery, etc.);
for blocking nerve plexuses (brachial lumbar, etc.);
for epidural and spinal anesthesia,
local anesthesia in ophthalmology.
As an antiarrhythmic drug, lidocaine hydrochloride is used in the treatment of mono- and polytopic extrasystoles of ventricular tachycardia (including those occurring during general anesthesia, during cardiac surgery, in case of overdose of cardiac glycosides, etc.), to relieve ventricular fibrillation in the acute phase of myocardial infarction, as well as in ventricular defibrillation as a component pharmacotherapy. In addition, it is used to prevent ventricular extrasystole and tachycardia of any origin.
Special instructions
Special instructions
Use with caution in patients with impaired liver function, circulatory failure, arterial hypotension, renal failure, epilepsy. In these cases, a reduction in the dose of the drug is required.
With rapid intravenous administration, a sharp decrease in blood pressure and the development of collapse can occur.
In these cases, mezaton, ephedrine and other vasoconstrictors are used. Lidocaine solutions should be carefully injected into highly vascularized tissues to avoid the drug entering the lumen of the vessel (for example, in the neck area during thyroid surgery) (in such cases, smaller doses of lidocaine are indicated.
The drug should be used with particular caution in the presence of injuries to the mucous membranes, with mental retardation, as well as in very old and/or weakened patients who are already receiving drugs such as lidocaine for cardiac problems.
In dentistry and orthopedics, the drug should be used only with elastic impression materials.
Avoid ingestion of the aerosol or contact with the eyes; it is important to prevent the aerosol from entering the respiratory tract (risk of aspiration). Applying the drug to the back of the throat requires special care. It should be remembered that Lidocaine suppresses the pharyngeal reflex and inhibits the cough reflex, which can lead to aspiration and bronchopneumonia.
Use in pediatrics
It should be borne in mind that in children the swallowing reflex occurs much more often than in adults.
Lidocaine in aerosol form is not recommended for local anesthesia before tonsillectomy and adenotomy in children under 8 years of age.
Impact on the ability to drive vehicles and operate machinery
If side effects after using the drug do not cause discomfort, there are no restrictions on driving vehicles or operating machinery.
Active ingredient
Active ingredient
Lidocaine
Composition
Composition
Active ingredient:
lidocaine hydrochloride;
Excipients:
sodium chloride,
sodium hydroxide 1 M solution to pH 5.0 – 7.0
water for injections
Contraindications
Contraindications
Sick sinus syndrome.
Severe bradycardia.
2nd and 3rd degree atrioventricular block (except when a probe is inserted to simulate the ventricles).
Cardiogenic shock.
Myasthenia.
Increased individual sensitivity to lidocaine.
History of epileptiform seizures caused by lidocaine.
Severe liver dysfunction.
When using plaster of Paris as an impression material in dentistry, the aerosol is contraindicated due to the risk of aspiration.
Side Effects
Side Effects
headache
dizziness
numbness of the tongue and oral mucosa
lowering blood pressure
decrease in heart rate
a burning sensation when the aerosol hits the anesthetized surface (stops a few seconds after the onset of anesthesia).
Interaction
Interaction
It is not advisable to combine lidocaine with the following drugs:
With beta-blockers due to increased toxic properties of lidocaine, with digitoxin – due to a weakening of the cardiotonic effect, with curare-like drugs – muscle relaxation increases.
It is irrational to prescribe lidocaine together with ajmaline, amiodarone, verapamil or quinidine due to increased cardiodepressive effects.
The combined use of lidocaine and procainamide can cause central nervous system stimulation and hallucinations.
With intravenous administration of hexenal or thiopental sodium against the background of the action of lidocaine, respiratory depression is possible.
Under the influence of MAO inhibitors, the local anesthetic effect of lidocaine is likely to be enhanced. Patients taking MAO inhibitors should not be prescribed parenteral lidocaine.
With the simultaneous administration of lidocaine and polymyxin-B, an increased inhibitory effect on neuromuscular transmission is possible, so in this case it is necessary to monitor the patient’s respiratory function.
With the simultaneous use of lidocaine with sleeping pills or sedatives, their inhibitory effect on the central nervous system may be enhanced. When lidocaine is administered intravenously to patients taking cimetidine, undesirable effects such as stunned state, drowsiness, bradycardia, parasthesia, etc. are possible. This is due to an increase in the level of lidocaine in the blood plasma, which is explained by the release of lidocaine from binding to blood proteins, as well as a slowdown in its inactivation in the liver. If combination therapy with these drugs is necessary, the dose of lidocaine should be reduced.
Pharmaceutical interaction
When used concomitantly, the following drugs increase the concentration of lidocaine in the blood serum: aminazine, cimetidine, propranolol, pethidine, bupivacaine, quinidine, disopyramide, amitriptyline, imipramine, nortriptyline.
Overdose
Overdose
Symptoms: symptoms from the central nervous system (including convulsions) and the cardiovascular system are possible.
Treatment: for symptoms from the central nervous system and the cardiovascular system, it is necessary to ensure the airway is open, provide access to fresh air, oxygen supply and/or artificial respiration. If convulsions occur, 50-100 mg of dithiline and/or 5-15 mg of diazepam should be administered as quickly as possible; it is possible to use barbituric acid derivatives (sodium thiopental). In the acute phase of lidocaine overdose, dialysis is ineffective.
For bradycardia and cardiac conduction disorders, atropine 0.5-1 mg IV can be prescribed.
Storage conditions
Storage conditions
In a dry place, protected from light.
Shelf life
Shelf life
2 years.
Manufacturer
Manufacturer
Biosynthesis, Russia
Additional information
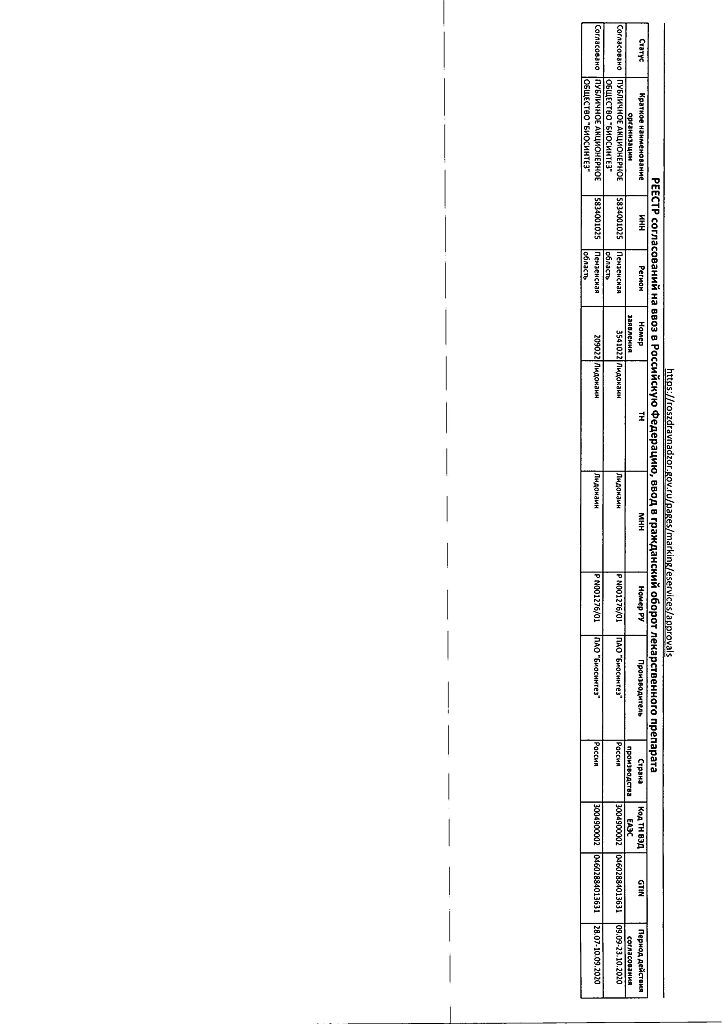
| Shelf life | 2 years. |
|---|---|
| Conditions of storage | In a dry place protected from light. |
| Manufacturer | Biosintez, Russia |
| Medication form | solution for injection |
| Brand | Biosintez |
Other forms…
Related products
Buy Lidocaine, 20 mg/ml 2 ml 10 pcs with delivery to USA, UK, Europe and over 120 other countries.