No products in the cart.
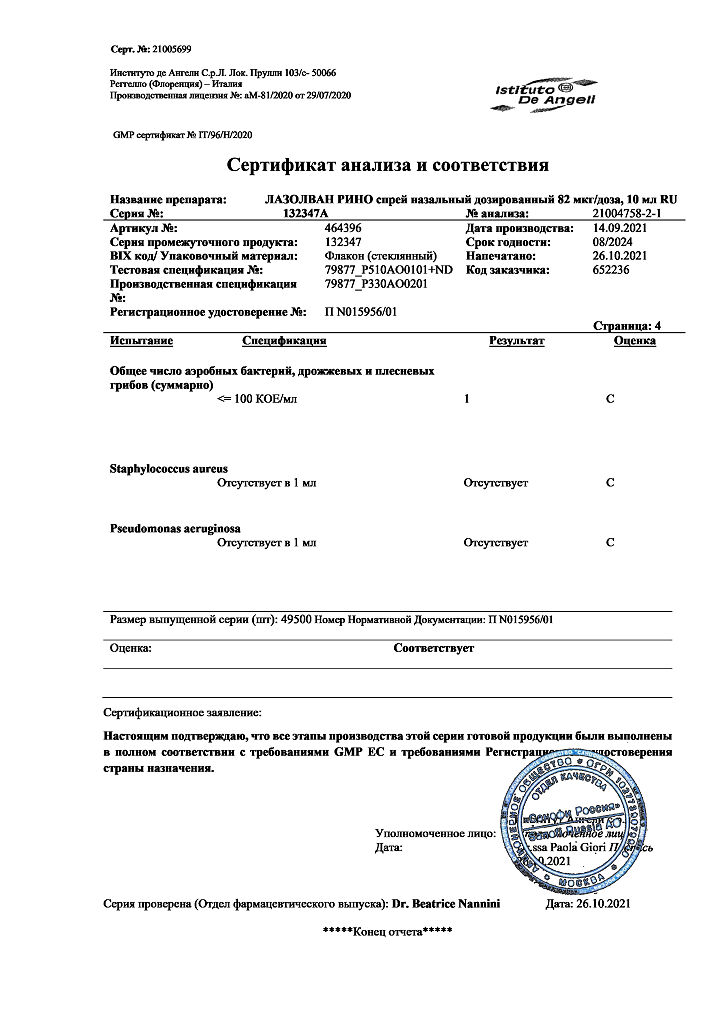
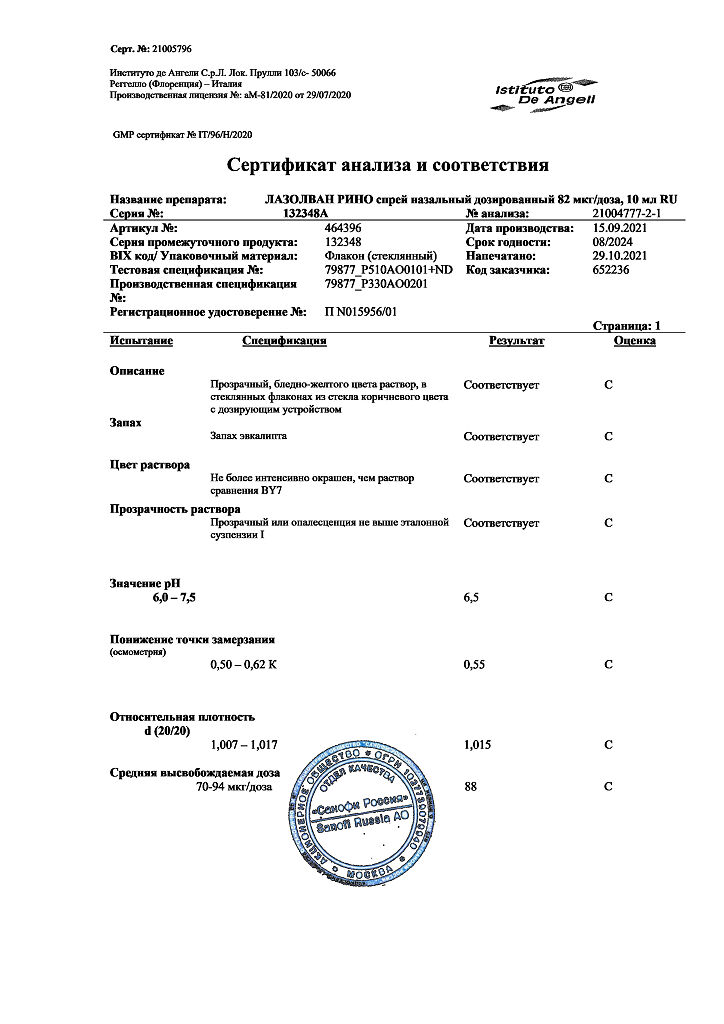
Lazolvan Rhino, 82 mcg/dose 10 ml
€11.37 €9.48
Description
The active substance of Lazolvan Rhino is tramazoline hydrochloride, alpha2-adrenomimetic, causes vasoconstriction. When applied to the nasal mucosa, the drug reduces swelling due to its vasoconstrictor effect. As a result, the patency of the nasal passages is quickly restored, and nasal breathing is relieved for a long time.
The action of the drug starts within the first 5 minutes and lasts for 8-10 hours.
Pharmacokinetic studies in humans have not been performed. The pharmacokinetics of tramazoline has been studied in rats, rabbits and monkeys. It has been shown that after administration of the drug orally or intranasally, 50-80% of the administered dose is absorbed. Tramazoline and its metabolites are distributed in all internal organs, Cmax is noted in the liver.
After oral or topical administration, the major metabolites are detected in the urine. The terminal T1/2 is 5 to 7 h.
Indications
Indications
Swelling of the nasal mucosa, nasal congestion caused by acute respiratory diseases and/or hay fever (rhinitis, hay fever).
Sinusitis and otitis media (eustacheitis), to facilitate the outflow of the contents of the paranasal sinuses, as recommended by a doctor.
Pharmacological effect
Pharmacological effect
The active substance of the drug Lazolvan Rino – tramazoline hydrochloride, an alpha2-adrenergic agonist, causes vasoconstriction. When applied to the mucous membranes of the nose, due to the vasoconstrictor effect, the drug reduces swelling. As a result, the patency of the nasal passages is quickly restored, and nasal breathing becomes easier for a long time.
The effect of the drug begins within the first 5 minutes and lasts 8-10 hours.
Pharmakinetic studies have not been conducted in humans. The pharmacokinetics of tramazolin have been studied in rats, rabbits and monkeys. It has been shown that after administration of the drug orally or intranasally, 50-80% of the administered dose is absorbed. Tramazolin and its metabolites are distributed in all internal organs, Cmax is noted in the liver.
After oral or topical administration, the main metabolites are determined in the urine. Terminal T1/2 ranges from 5 to 7 hours.
Special instructions
Special instructions
If after 7 days of taking the drug there is no positive trend in symptoms, you should consult a doctor to decide whether to stop taking the drug or continue treatment.
Long-term use of nasal vasoconstrictors can lead to the development of chronic inflammation and nasal congestion, as well as atrophy of the nasal mucosa.
Avoid contact of the drug with the eyes.
Impact on the ability to drive vehicles and other mechanisms that require increased concentration
Studies on the effect of the drug on the ability to drive a car and operate machinery have not been conducted. However, when taking the drug, undesirable effects such as hallucinations, drowsiness, sedation, dizziness and fatigue are possible. Therefore, care must be taken when operating vehicles and machinery. If the above side effects occur, you should avoid performing potentially hazardous tasks such as driving or operating machinery.
Active ingredient
Active ingredient
Tramazolin
Composition
Composition
1 dose contains:
Active substance:
tramazoline hydrochloride (in the form of tramazoline hydrochloride monohydrate) 82 mcg;
Excipients:
citric acid monohydrate – 270 mcg,
sodium hydroxide – 154 mcg,
benzalkonium chloride – 14 mcg,
hypromellose (hydroxypropyl methylcellulose) – 35 mcg,
povidone – 2101 mcg,
glycerol 85% – 700 mcg,
magnesium sulfate heptahydrate – 49 mcg,
magnesium chloride hexahydrate – 35 mcg,
calcium chloride dihydrate – 11 mcg,
sodium bicarbonate – 1 mcg,
sodium chloride – 183 mcg,
cineole (eucalyptol) – 7 mcg,
levomenthol (L-menthol) – 14 mcg,
racemic camphor – 14 mcg,
purified water – up to 66358 mcg.
Contraindications
Contraindications
Hypersensitivity to tramazoline hydrochloride or benzalkonium hydrochloride, as well as other components of the drug;
angle-closure glaucoma;
atrophic rhinitis;
history of cranial surgery performed through the nasal cavity;
children under 6 years of age.
With caution: simultaneous use of MAO inhibitors, tricyclic antidepressants, vasopressor and antihypertensive drugs; patients with arterial hypertension, heart disease, hyperthyroidism, prostatic hypertrophy, pheochromocytoma, porphyria should use Lazolvan® Rino only on the recommendation of a doctor due to the potential risk of systemic absorption of the drug.
Side Effects
Side Effects
From the nervous system: rarely – dizziness, taste disturbances; infrequently – headache; frequency not established – drowsiness, sedation.
Mental disorders: infrequently – anxiety; frequency not established – hallucinations, insomnia.
From the cardiovascular system: infrequently – palpitations; frequency not established – arrhythmia, tachycardia, increased blood pressure.
From the respiratory system, chest and mediastinum: often – nasal discomfort; uncommon – nasal swelling, dry nose, rhinorrhea, sneezing; rarely – nosebleeds.
From the gastrointestinal tract: infrequently – nausea.
From the immune system: frequency not established – hypersensitivity.
From the skin and subcutaneous tissues: frequency has not been established – rash, itching, swelling of the skin.
General and local disorders: frequency not established – swelling of the mucous membranes, fatigue.
Interaction
Interaction
Some antidepressants (MAO inhibitors and tricyclic antidepressants) and vasoconstrictors, when administered simultaneously, can cause an increase in blood pressure.
Combined use with tricyclic antidepressants can lead to the development of arrhythmia.
Concomitant use with antihypertensive drugs (especially those that affect the sympathetic nervous system) can lead to various cardiovascular effects.
Overdose
Overdose
Symptoms: following an increase in blood pressure and tachycardia, it is possible (especially in children) for a fall in blood pressure, the development of shock, reflex bradycardia, and a decrease in body temperature. By analogy with other alpha-adrenergic agonists, the clinical picture of intoxication may be unclear, since the phases of stimulation and depression of the central nervous system and cardiovascular system can replace each other. Especially in children, intoxication leads to effects on the central nervous system: symptoms of central nervous system stimulation include anxiety, agitation, hallucinations and seizures; Symptoms of central nervous system depression include decreased body temperature, lethargy, drowsiness, and coma.
In addition, the following symptoms may develop: mydriasis, miosis, increased sweating, fever, pallor, cyanosis of the lips, dysfunction of the cardiovascular system (including bradycardia and cardiac arrest); respiratory failure (including respiratory failure, respiratory arrest); mental disorders.
Treatment: In case of nasal overdose, rinse or clean the nose immediately. Symptomatic therapy may be required.
Storage conditions
Storage conditions
At a temperature not exceeding 25 °C
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Instituto de Angeli S.r.L., Italy
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | At a temperature not exceeding 25 °C |
| Manufacturer | Instituto de Angeli S.r.l., Italy |
| Medication form | dosed nasal spray |
| Brand | Instituto de Angeli S.r.l. |
Related products
Buy Lazolvan Rhino, 82 mcg/dose 10 ml with delivery to USA, UK, Europe and over 120 other countries.