No products in the cart.
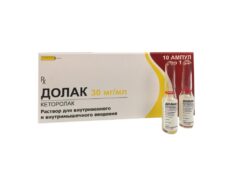
Ketorolac, 30 mg/ml 1 ml 10 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
Ketorolac has a pronounced analgesic effect, also has anti-inflammatory and moderate antipyretic effects.
The mechanism of action is associated with non-selective inhibition of cyclooxygenase 1 and 2 enzyme activity, mainly in peripheral tissues, the consequence of which is inhibition of prostaglandin biosynthesis – modulators of pain sensitivity, thermoregulation and inflammation. Ketorolac is a racemic mixture of [-]S and [+]R enantiomers, with analgesic effect due to the [-]S form.
It is comparable to morphine in its analgesic effect and is considerably superior to other non-steroidal anti-inflammatory drugs.
The drug does not affect opioid receptors, does not depress respiration, does not cause drug dependence, does not have sedative and anxiolytic effects. After intramuscular administration the onset of analgesic effect is observed after 0.5 hours, the maximum effect is reached after 1-2 hours.
Pharmacokinetics
. When administered orally, Ketorolac is well absorbed in the gastrointestinal tract – maximum concentration (Cmax) in blood plasma (0.7-1.1 mcg/ml) is reached 40 min after an empty stomach dose of 10 mg. Fat-rich food decreases the maximum blood concentration of the drug and delays its achievement by 1 hour. 99% of the drug is bound to plasma proteins and in case of hypoalbuminemia the amount of free substance in blood increases.
The bioavailability is 80-100%. Time of reaching equilibrium concentration (Ссs) by oral administration – 24 hours when administered 4 times a day (higher than subtherapeutic) and is 0.39-0.79 mcg/ml after 10 mg oral administration. The volume of distribution is 0.15-0.33 l/kg.
In patients with renal insufficiency the distribution volume of the drug may increase 2-fold, and the distribution volume of its R-enantiomer – by 20%.
Transfers to breast milk: when the mother takes 10 mg of ketorolac, Cmax in milk is reached 2 hours after the first dose and is 7.3 ng/ml; 2 hours after the second dose of ketorolac (when using the drug 4 times a day) it is 7.9 ng/ml. More than 50% of the administered dose is metabolized in the liver to form pharmacologically inactive metabolites.
The main metabolites are glucuronides, which are excreted by the kidneys and p-hydroxyketorolac. It is excreted 91% by the kidneys and 6% through the intestine.
The elimination half-life (T1/2) in patients with normal renal function is on average 5.3 h. T1/2 is longer in elderly patients and shorter in younger patients. Liver function has no effect on T1/2. In patients with impaired renal function with plasma creatinine concentration of 19-50 mg/l (168-442 μmol/l). T1/2 is 10.3-10.8 hours, in more severe renal failure more than 13.6 hours. It is not excreted by hemodialysis.
Indications
Indications
Pain syndrome of severe to moderate severity:
Active ingredient
Active ingredient
Composition
Composition
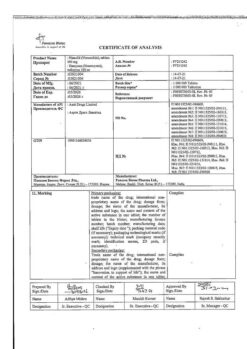
1 ml of solution for injection contains:
the active ingredient:
ketorolaca trometamol (ketorolaca trometamine) – 30 mg.
How to take, the dosage
How to take, the dosage
Inject deep into the muscle, slowly (or by intravenous stream) for at least 15 seconds in minimum effective doses tailored to the pain intensity and the patient’s response.
If necessary, opioid analgesics in reduced doses may be administered at the same time.
The single dose when administered intramuscularly or intravenously: – in adults under 65 years of age and children over 16 years – 10-30 mg depending on the severity of the pain syndrome, – in adults over 65 years or with impaired renal function – 10-15 mg.
Doses with multiple parenteral administration: Intramuscular – Adults under 65 years of age and children over 16 years of age are given 10-60 mg on the first administration, then 10-30 mg every 6 hours (usually 30 mg every 6 hours), – Adults over 65 years of age or with renal dysfunction are given 10-15 mg every 4-6 hours; intravenously – adults under 65 years of age and children over 16 years of age are given 10-30 mg by jet, then 10-30 mg every 6 hours, with a continuous infusion using an infusometer, the initial dose is 30 mg and then the infusion rate is 5 mg/h. – adults over 65 years of age or with renal dysfunction are given 10-15 mg every 6 hours by jet.
The maximum daily dose for adults under 65 years of age and children over 16 years of age should not exceed 90 mg, and for adults over 65 years of age or with impaired renal function, 60 mg for both intramuscular and intravenous routes of administration. Continuous intravenous infusion should not last more than 24 hours.
If parenteral administration, the duration of treatment should not exceed 5 days.
When changing from parenteral administration of the drug to oral administration, the cumulative daily dose of both dosage forms on the day of transfer must not exceed 90 mg for adults under 65 and children over 16 and 60 mg for adults over 65 or with impaired renal function. At the same time, the dose of the drug in tablets on the day of transfer should not exceed 30 mg.
Interaction
Interaction
Concomitant use of ketorolac with acetylsalicylic acid or other nonsteroidal anti-inflammatory drugs, calcium preparations, glucocorticosteroids, ethanol, corticotropin may lead to gastrointestinal ulcers and development of gastrointestinal bleeding.
Co-administration with paracetamol increases nephrotoxicity, with methotrexate – hepato- and nephrotoxicity. Co-administration of ketorolac and methotrexate is possible only with the use of low doses of the latter (monitor the plasma concentration of methotrexate).
The use of ketorolac may decrease clearance of methotrexate and lithium and increase toxicity of these substances. Concomitant administration with indirect anticoagulants, heparin, thrombolytics, antiaggregants, cefoperazone, cefotetan and pentoxifylline increases the risk of bleeding.
Limits the effect of hypotensive and diuretic drugs (reduces the synthesis of prostaglandins in the kidneys). When combining with opioid analgesics, the doses of the latter may be significantly reduced, since their effects are enhanced. Hypoglycemic effect of insulin and oral hypoglycemic agents increases (recalculation of the dose is necessary).
The co-administration with valproic acid causes impairment of platelet aggregation. Increases plasma concentrations of verapamil and nifedipine.
When prescribed with other nephrotoxic drugs (including gold drugs) the risk of nephrotoxicity increases. Probenecid and drugs that block tubular secretion decrease clearance of ketorolac and increase its concentration in blood plasma.
The solution for injection should not be mixed in the same syringe with morphine sulfate, promethazine and hydroxyzine due to precipitation.
Pharmaceutically incompatible with tramadol solution, lithium preparations. Solution for injection is compatible with 0.9% sodium chloride solution, 5% dextrose solution, Ringer and Ringer-lactate solutions, “Plasmalite” solution, as well as with infusion solutions containing aminophylline, lidocaine hydrochloride, dopamine hydrochloride, human insulin of short action and heparin sodium salt.
Special Instructions
Special Instructions
When combined with other nonsteroidal anti-inflammatory drugs, fluid retention, decompensation of cardiac activity, arterial hypertension may be observed. The effect on platelet aggregation stops after 24-48 hours.
Hypovolemia increases the risk of adverse reactions from the kidneys. If necessary, can be administered in combination with narcotic analgesics.
Ketorolac is not recommended for premedication, maintenance anesthesia and analgesia in obstetric practice.
Do not use simultaneously with paracetamol for more than 5 days. Patients with blood clotting disorders are prescribed the drug only with continuous monitoring of platelet count, especially in the postoperative period, which requires close monitoring of hemostasis.
It is recommended to avoid performing work requiring increased attention and quick reactions (driving, working with machinery, etc.).
Contraindications
Contraindications
Side effects
Side effects
Frequently – more than 3%, less frequently – 1-3%, rarely – less than 1%.
The digestive system: often (especially in elderly patients over 65 years of age with a history of gastrointestinal erosive ulcers) – gastralgia, diarrhea; less frequently – stomatitis, flatulence, constipation, vomiting, feeling of stomach fullness; rarely – nausea, erosive ulcers of the gastrointestinal tract (including perforation and/or bleeding – abdominal pain, spasm or burning of the epigastric region.with perforation and/or bleeding – abdominal pain, spasm or burning in epigastric region, blood in stool or melena, vomiting with blood or “coffee grounds” type, nausea, heartburn, etc.), cholestatic jaundice, hepatitis, hepatomegaly, acute pancreatitis.
Urinary system disorders: rare – acute renal failure, lumbar pain with or without hematuria and/or azotemia, hemolytic-uremic syndrome (hemolytic anemia, renal failure, thrombocytopenia, purpura), frequent urination, increased or decreased volume of urine, nephritis, edema of renal genesis.
Sense organs: rarely – hearing loss, tinnitus, visual impairment (including blurred vision).
Respiratory system disorders: rarely – bronchospasm or dyspnea, rhinitis, pulmonary edema, laryngeal edema (shortness of breath, difficulty in breathing).
CNS disorders: often – headache, dizziness, somnolence, rarely – aseptic meningitis (fever, severe headache, cramps, neck and/or back stiffness), hyperactivity (mood changes, anxiety), hallucinations, depression, psychosis.
Cardiovascular system: less frequently – increase in blood pressure, rarely – fainting.
Hematopoietic system: rarely – anemia, eosinophilia, leukopenia.
Hemostatic system disorders: rare – postoperative wound bleeding, nasal bleeding, rectal bleeding.
Skin disorders: less frequently – skin rash (including maculopapular rash), purpura, rarely – exfoliative dermatitis (fever with or without chills, redness, thickening or peeling of the skin, swelling and/or pain of the palatine tonsils), urticaria, Stevens-Johnson syndrome, Lyell syndrome.
Local reactions: less frequent – burning or pain at the injection site.
Allergic reactions: rarely – anaphylaxis or anaphylactoid reactions (changes in complexion, skin itching, tachypnea or dyspnea, eyelid edema, periorbital edema, shortness of breath, difficulty in breathing, heaviness in the chest, wheezing).
Others: often – edema (face, shins, ankles, fingers, feet, weight gain); less often – increased sweating, rarely – tongue swelling, fever.
Overdose
Overdose
Symptoms (single administration): abdominal pain, nausea, vomiting, gastrointestinal erosive and ulcerative lesions, impaired renal function, metabolic acidosis.
Treatment: symptomatic (maintenance of vital body functions). Dialysis is ineffective.
Similarities
Similarities
Additional information
| Shelf life | 2 years. |
|---|---|
| Conditions of storage | Store in a light-protected place at 15 to 25 °C. Keep out of reach of children. |
| Manufacturer | Sintez OAO, Russia |
| Medication form | solution |
| Brand | Sintez OAO |
Other forms…
Related products
Buy Ketorolac, 30 mg/ml 1 ml 10 pcs with delivery to USA, UK, Europe and over 120 other countries.