No products in the cart.
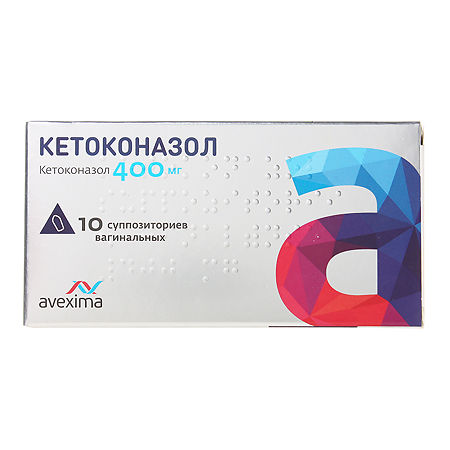
Ketoconazole, vaginal suppositories 400 mg 10 pcs
€17.47 €14.56
Description
The active ingredient is ketoconazole, a derivative of imidazoldioxolane.
It has fungicidal and fungostatic action against dermatophytes (Trichophyton spp., Epidermophyton floccosum, Microsporum spp.) and yeast fungi (Pityrosporum spp., Candida spp. (including Candida albicans).
The mechanism of action is inhibition of ergosterol biosynthesis and changing lipid composition of the membrane of fungi.
The drug is active against staphylococci and streptococci.
Indications
Indications
Treatment of acute and chronic recurrent vaginal mycosis.
Prevention of the occurrence of fungal infections of the vagina with reduced body resistance and during treatment with antibacterial agents and other drugs that disrupt the normal microflora of the vagina.
Pharmacological effect
Pharmacological effect
The active substance is ketoconazole, an imidazoledioxolane derivative.
It has a fungicidal and fungostatic effect against dermatophytes (Trichophyton spp., Epidermophyton floccosum, Microsporum spp.) and yeast fungi (Pityrosporum spp., Candida spp. (including Candida albicans).
The mechanism of action is to inhibit the biosynthesis of ergosterol and change the lipid composition of the fungal membrane.
The drug is active against staphylococci and streptococci.
Special instructions
Special instructions
During treatment, it is necessary to regularly monitor the peripheral blood picture, the functional state of the liver and kidneys.
Contact with latex preparations (contraceptive diaphragms, condoms) should be avoided.
Active ingredient
Active ingredient
Ketoconazole
Composition
Composition
1 suppository contains:
active substance:
ketoconazole 400 mg;
excipients:
butylated hydroxyanisole 0.5 mg,
semisynthetic glycerides (Suppotsir AM) – sufficient quantity to obtain a suppository weighing 2000 mg.
Contraindications
Contraindications
Hypersensitivity to the components of the drug, pregnancy, lactation.
Side Effects
Side Effects
Hyperemia and irritation of the vaginal mucosa, vaginal itching.
Allergic reactions: skin rash, urticaria.
Interaction
Interaction
When used simultaneously with rifampicin and isoniazid, the plasma concentration of ketoconazole decreases. When used simultaneously with cyclosporine, indirect anticoagulants and methylprednisolone, ketoconazole may increase the concentration of the latter in plasma.
Ethanol and other hepatotoxic drugs increase the risk of developing hepatic parenchyma. Increases the concentration of sulfonylurea derivatives and increases the risk of hypoglycemia.
Weakens the effect of amphotericin B. Increases plasma concentrations of digoxin, midazolam and triazolam. Increases the bioavailability of indinavir.
Reduces the stimulating effect of corticotropin on the adrenal glands. When combined with terfenadine, astemizole and cisapride, it increases the risk of severe ventricular tachycardia, incl. “pirouette” type.
Increases the risk of breakthrough bleeding with simultaneous use of oral contraceptives with low hormone content. Increases the toxicity of phenytoin.
Overdose
Overdose
Due to the low absorption of the drug when used intravaginally in recommended (therapeutic) doses, an overdose is unlikely.
If the drug is accidentally ingested, symptoms of poisoning may occur.
There is no specific antidote.
If necessary, take activated carbon and carry out symptomatic treatment.
Storage conditions
Storage conditions
In a place protected from light, at a temperature not exceeding 25 °C.
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Pharmaprim/Avexima Siberia, Russia
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In a light-protected place at a temperature not exceeding 25 °C. |
| Manufacturer | Avexima Siberia, Russia |
| Medication form | vaginal suppositories |
| Brand | Avexima Siberia |
Other forms…
Related products
Buy Ketoconazole, vaginal suppositories 400 mg 10 pcs with delivery to USA, UK, Europe and over 120 other countries.