No products in the cart.
Haloperidol-Ratiofarm, drops 2 mg/ml 30 ml
€3.47 €3.08
Out of stock
(E-mail when Stock is available)
Description
Pharmacotherapeutic group: antipsychotic (neuroleptic).
ATX code: N05AD01
Pharmacological action
Pharmacodynamics
Antipsychotic (neuroleptic), butyrophenone derivative. It has strong antipsychotic effect, blocks postsynaptic dopamine receptors in mesolimbic and mesocortical structures of the brain. Inhibits mediator release by reducing the permeability of presynaptic membranes. High antipsychotic activity is combined with a moderate sedative effect (in small doses has an activating effect) and a pronounced antiemetic effect. It causes extrapyramidal disorders and has practically no choline-blocking effect.
The sedative action is caused by the blockade of alpha-adrenoreceptors of reticular formation in brain stem; the antiemetic action – by the blockade of dopamine D2-receptors of trigger zone of vomiting center; hypothermic action and galactorea – by the blockade of dopamine receptors of hypothalamus. Prolonged use is accompanied by changes in endocrine status; prolactin production increases in the anterior pituitary lobe and gonadotropic hormones production decreases.
Removes persistent changes in personality, delirium, hallucinations, delusions, and increases interest in the environment.
Pharmacokinetics
Absorption when taken orally is 60%. Binding with blood plasma proteins – 92%. Maximal concentration in plasma is reached when administered orally after 3 hours, after intramuscular injection – after 10-20 minutes. The volume of distribution is 15-35 l/kg, the binding to plasma proteins is 92%. It easily passes through histohematic barriers, including the blood-brain barrier.
Metabolized in the liver, subjected to the effect of “first passage” through the liver. Excreted with bile and urine: after per oral administration 15% is excreted with bile, 40% – with urine (including 1% – in unchanged form). Metabolism of haloperidol is accelerated by enzyme-inducing substances (phenobarbital, phenytoin and carbamazepine).
Because of the large volume of distribution and low plasma concentration, only a very small amount of haloperidol is removed by dialysis. It penetrates into breast milk. CYP2D6, CYP3A3, CYP3A5, CYP3A7 isoenzymes are involved in metabolism of haloperidol. It is an inhibitor of CYP2D6. There are no active metabolites. The elimination half-life when taken orally averages 24 hours (15 to 37 hours).
Indications
Indications
Acute psychotic syndromes, accompanied by delusions, hallucinations, disorders of thinking and consciousness, catatonic syndromes, delirium and other exogenous psychotic syndromes.
Chronic endogenous and exogenous psychoses (suppression of symptoms and prevention of relapses).
Psychomotor agitation.
Vomiting, stuttering, if other therapy is impossible or resistance to treatment.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: antipsychotic (neuroleptic).
ATX code: N05AD01
Pharmacological action
Pharmacodynamics
Antipsychotic drug (neuroleptic), butyrophenone derivative. It has a pronounced antipsychotic effect, blocks postsynaptic dopamine receptors in the mesolimbic and mesocortical structures of the brain. Inhibits the release of mediators, reducing the permeability of presynaptic membranes. High antipsychotic activity is combined with a moderate sedative effect (in small doses it has an activating effect) and a pronounced antiemetic effect. Causes extrapyramidal disorders and has virtually no anticholinergic effect.
The sedative effect is due to the blockade of alpha-adrenergic receptors in the reticular formation of the brain stem; antiemetic effect – blockade of dopamine D2 receptors of the trigger zone of the vomiting center; hypothermic effect and galactorrhea – blockade of dopamine receptors of the hypothalamus. Long-term use is accompanied by a change in endocrine status; in the anterior lobe of the pituitary gland, the production of prolactin increases and the production of gonadotropic hormones decreases.
Eliminates persistent personality changes, delusions, hallucinations, mania, and increases interest in the environment.
Pharmacokinetics
Absorption when taken orally – 60%. Communication with blood plasma proteins – 92%. The maximum concentration in plasma is achieved after oral administration after 3 hours, after intramuscular administration after 10-20 minutes. Volume of distribution – 15-35 l/kg, binding to plasma proteins – 92%. Easily passes through histohematic barriers, including the blood-brain barrier.
Metabolized in the liver, undergoes a “first pass” effect through the liver. Excreted in bile and urine: after oral administration, 15% is excreted in bile, 40% in urine (including 1% unchanged). The metabolism of haloperidol is accelerated by enzyme-inducing substances (phenobarbital, phenytoin and carbamazepine).
Due to its large volume of distribution and low plasma concentration, only a very small amount of haloperidol is removed by dialysis. Passes into breast milk. The isoenzymes CYP2D6, CYP3A3, CYP3A5, CYP3A7 are involved in the metabolism of haloperidol. It is an inhibitor of CYP2D6. There are no active metabolites. The half-life when taken orally is on average 24 hours (from 15 to 37 hours).
Special instructions
Special instructions
In rare cases, sudden death has been reported in patients with mental illness receiving antipsychotic drugs, including haloperidol (see section “Side effects”).
If fever, gingivitis and stomatitis, pharyngitis, purulent sore throat and flu-like symptoms occur, especially in the first three months of treatment, you should immediately consult your doctor. Self-medication with analgesics should be avoided.
Before starting treatment with haloperidol, patients need to undergo a complete blood count (including a differentiated count of blood cells and platelet counts), ECG and electroencephalogram parameters. If deviations from the norm are detected, haloperidol can be used only according to absolute indications and subject to mandatory control studies of general blood parameters. Before starting treatment and during treatment, monitoring of electrolyte balance and correction of hypokalemia if necessary is necessary.
In patients with organic brain damage, arteriosclerotic cerebrovascular diseases and endogenous depression, special caution is required when treating with haloperidol.
Haloperidol may precipitate seizures in patients with epilepsy and other conditions predisposing them to seizures.
Neuroleptic malignant syndrome may occur during treatment with haloperidol. Hyperthermia is often an early sign of this syndrome. If neuroleptic malignant syndrome is suspected, the drug should be immediately discontinued and appropriate therapy should be prescribed.
Due to the fact that elderly patients and patients with concomitant diseases of the cardiovascular system may experience intracardiac conduction disturbances, regular monitoring of cardiac activity is recommended during therapy. Patients with pheochromocytoma, renal failure, heart failure or cerebral insufficiency may develop hypotensive reactions during therapy with haloperidol, so such patients require careful monitoring.
During treatment with the drug, extrapyramidal symptoms (for example, tremor, rigidity, akathisia) may occur, and increasing the dose may lead to aggravation of these symptoms.
Although the prevalence of tardive dyskinesia has not been well studied, older patients (especially older women) are prone to developing this condition. The risk of developing tardive dyskinesia, especially the irreversible type, increases with increasing duration of treatment and with the use of antipsychotic doses. Tardive dyskinesia can also develop after short-term treatment in low doses. Early antipsychotic treatment may mask symptoms of primary tardive dyskinesia. If symptoms of tardive dyskinesia appear, it is necessary to evaluate the possibility of discontinuing all antipsychotic drugs, including haloperidol.
In patients suffering from psychosis with a predominance of depression, simultaneous use with antidepressants is recommended.
In patients suffering from bipolar disorder with manic episodes, there is a possibility of switching from manic episodes to episodes of depression. Monitoring of these patients is necessary, because the development of such manifestations of depression as suicidal behavior is possible.
Symptoms may appear after discontinuation of the drug.
Caution must be exercised when performing heavy physical work or taking a hot bath (heat stroke may develop due to suppression of central and peripheral thermoregulation in the hypothalamus).
During treatment, you should not take “anti-cold” over-the-counter medications (there may be an increase in m-anticholinergic effects and the risk of heat stroke).
Exposed skin should be protected from excess solar radiation due to the increased risk of developing photosensitivity reactions.
Treatment is stopped gradually to avoid the occurrence of withdrawal syndrome. Antiemetic effects may mask signs of drug toxicity and complicate the diagnosis of these conditions, the first symptom of which is nausea. Impact on the ability to drive vehicles and machinery
During the treatment period, it is necessary to avoid (especially in the first stages of treatment) or be careful when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Haloperidol
Composition
Composition
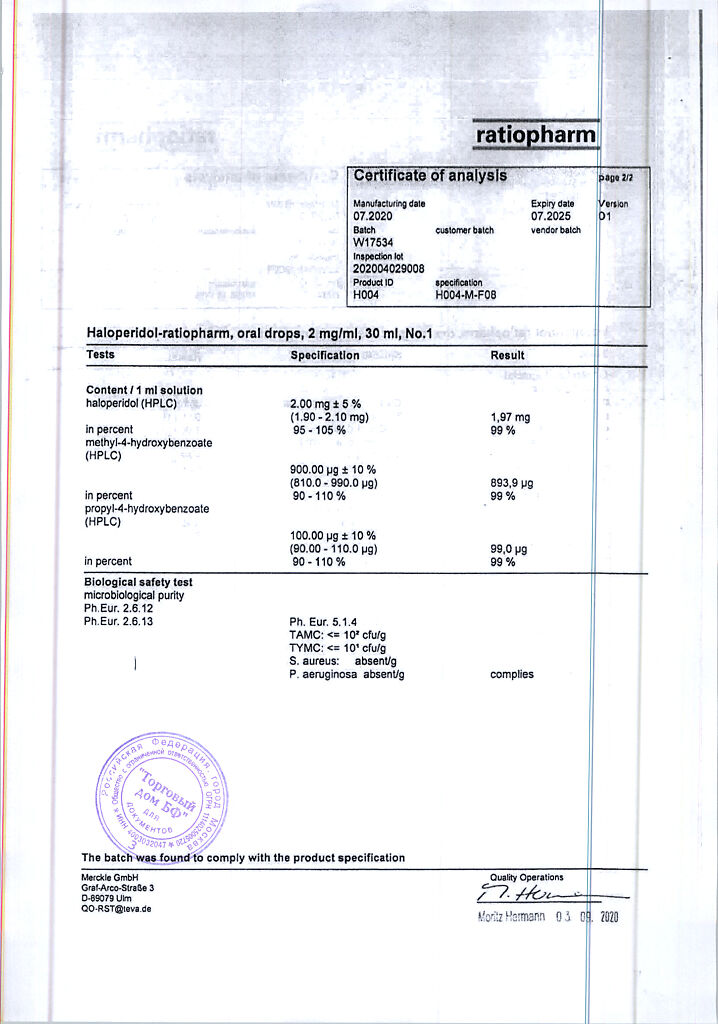
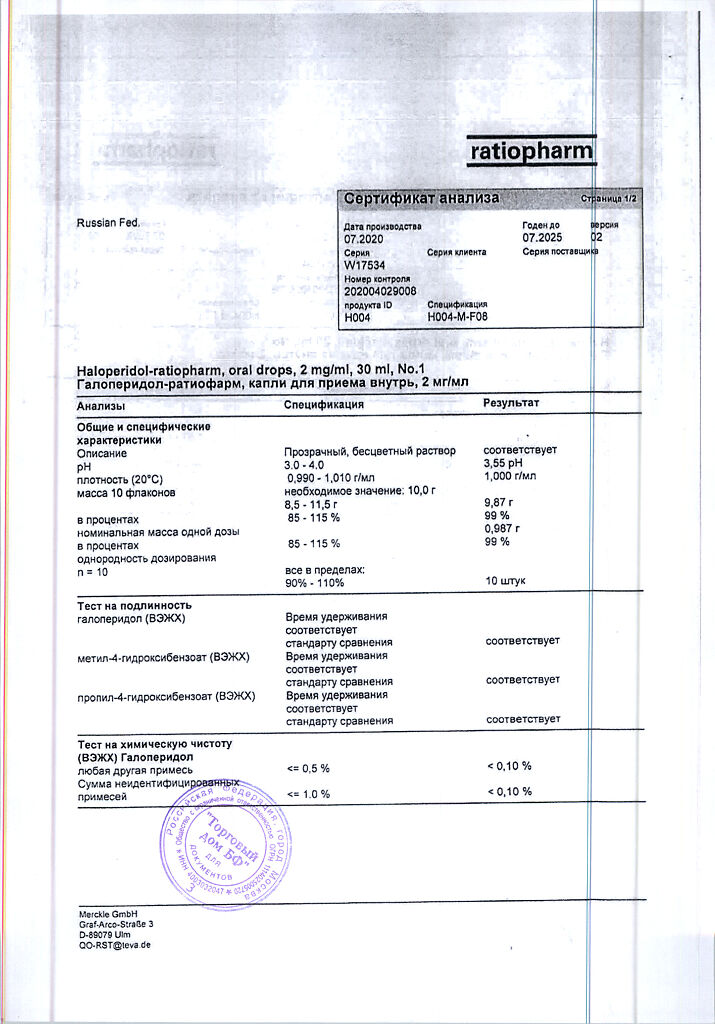
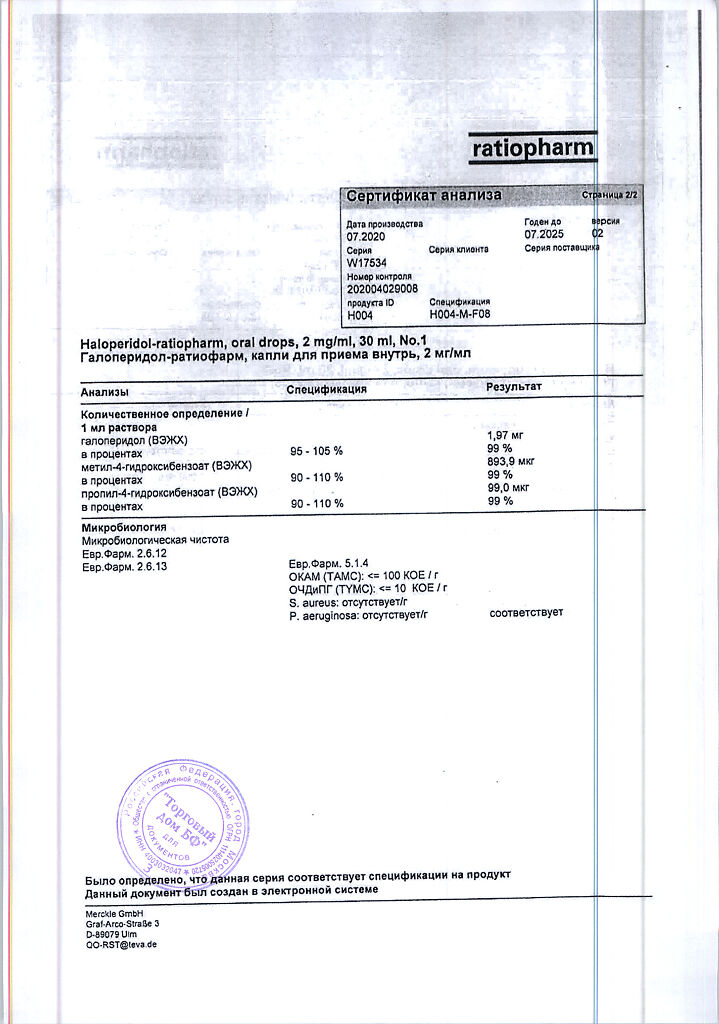
1 ml contains: active substance haloperidol 2,000 mg; excipients: methyl parahydroxybenzoate 0.900 mg; propyl parahydroxybenzoate 0.100 mg; lactic acid 1,700 mg; purified water 997,000 mg.
Pregnancy
Pregnancy
Pregnancy
The use of Haloperidol-ratiopharm during pregnancy is contraindicated. A pregnancy test should be performed before starting treatment. During treatment with the drug, it is necessary to use effective methods of contraception.
Breast-feeding
Haloperidol is excreted in breast milk. Haloperidol has been detected in small quantities in the plasma and urine of breastfed neonates of mothers treated with haloperidol. There is insufficient information on the effect of the drug on the development of newborns. The use of Haloperidol-ratiopharm during breastfeeding is contraindicated.
Fertility
Haloperidol increases prolactin levels. Hyperprolactinemia may inhibit the secretion of gonadotropin-releasing hormone from the hypothalamus, resulting in decreased secretion of pituitary gonadotropin. This phenomenon can suppress reproductive function by disrupting the formation of sex steroid hormones in both women and men (see section “Special Instructions”).
Contraindications
Contraindications
Hypersensitivity to haloperidol and/or other butyrophenone derivatives or other excipients of the drug;
coma of various origins;
children under 3 years of age;
pregnancy;
breastfeeding period;
severe depression of central nervous system (CNS) function;
diseases of the central nervous system accompanied by pyramidal or extrapyramidal symptoms (including Parkinson’s disease);
dementia with Lewy bodies;
progressive supranuclear palsy;
acquired or congenital prolongation of the QT interval;
acute myocardial infarction;
decompensated chronic heart failure;
history of ventricular arrhythmia or ventricular tachycardia of the “pirouette” type (torsade de pointes);
refractory hypokalemia;
simultaneous use with drugs that prolong the QT interval (see section “Interaction with other drugs”).
With caution
Acute intoxication with alcohol, narcotic analgesics, sleeping pills or psychotropic drugs that depress the central nervous system; liver and kidney failure; hypokalemia; bradycardia; simultaneous use of drugs that may contribute to the development of hypokalemia; other clinically significant cardiac disorders (for example, intracardiac conduction disorders, arrhythmias); prolactin-dependent tumors (for example, breast tumors); severe arterial hypotension or orthostatic dysregulation; endogenous depression; diseases of the hematopoietic system; history of neuroleptic malignant syndrome; organic brain diseases; epilepsy; hyperthyroidism.
Particular caution is required in patients with neurological symptoms of damage to the subcortical structures of the brain and a lowered seizure threshold (in history or during cessation of alcohol intake), since haloperidol reduces the seizure threshold, so “grand mal” type seizures may be observed. Patients with epilepsy should be treated with haloperidol only if anticonvulsant therapy is continued.
Haloperidol should not be used in severe depressive conditions. For concomitant depression and psychosis, haloperidol should be combined with antidepressants.
Since thyroxine may increase the incidence of adverse reactions associated with haloperidol, patients with hyperthyroidism should not receive treatment with haloperidol unless they are receiving adequate antithyroid therapy.
Side Effects
Side Effects
When using a dose of 1-2 mg/day, adverse reactions are not pronounced and are transient in nature. When using higher doses, the incidence of adverse reactions increases.
Within systemic organ classes, the following categories are used according to the frequency of occurrence of adverse reactions: very often (≥ 1/10); often (≥ 1/100, < 1/10); uncommon (≥ 1/1000, < 1/100); rare (≥ 1/10000, < 1/1000); very rare (< 1/10000); frequency unknown (cannot be estimated from available data).
From the blood and lymphatic system: infrequently – leukopenia; frequency unknown – pancytopenia, agranulocytosis, thrombocytopenia, neutropenia.
From the immune system: infrequently – hypersensitivity; frequency unknown – anaphylactic reactions.
From the endocrine system: rarely – hyperprolactinemia; frequency unknown – inadequate secretion of antidiuretic hormone.
Metabolic and nutritional disorders: frequency unknown – hypoglycemia.
From the mental side: very often – agitation, insomnia; often – psychotic disorders, depression; uncommon – confusion, loss of libido, decreased libido, feeling of anxiety.
From the nervous system: often – extrapyramidal disorders, headache, hyperkinesia; often – tardive dyskinesia, akathisia, bradykinesia, dyskinesia, dystonia, hypokinesia, hypertonicity, dizziness, drowsiness, tremor; uncommon – convulsions, parkinsonism, sedation, involuntary muscle contractions; rarely – neuroleptic malignant syndrome, impaired motor function, nystagmus; frequency unknown – akinesia, “mask-like” face, “cogwheel” rigidity, anxiety.
From the heart: infrequently – tachycardia; frequency unknown – ventricular fibrillation, torsade de pointes arrhythmia, ventricular tachycardia, extrasystole.
Vascular disorders: often – arterial hypotension, orthostatic hypotension.
From the respiratory system: infrequently – shortness of breath; rarely – bronchospasm, pharyngeal edema, laryngospasm.
From the digestive system: often – dryness of the oral mucosa, hypersecretion of the salivary glands, nausea, vomiting, constipation.
From the liver and biliary tract: often – changes in laboratory parameters of liver function; uncommon – hepatitis, jaundice; frequency unknown – acute liver failure, cholestasis.
From the skin and subcutaneous tissue: often – rash, infrequently – photosensitivity reaction, urticaria, itching, hyperhidrosis; frequency unknown – angioedema, exfoliative dermatitis, leukocytoclastic vasculitis.
From the musculoskeletal system: infrequently – torticollis, muscle rigidity, muscle spasm, musculoskeletal stiffness; rarely – spasm of the masticatory muscles (trismus), muscle twitching; frequency unknown – rhabdomyolysis.
From the genitourinary system: often – urinary retention.
Pregnancy, postpartum period, perinatal period: frequency unknown – withdrawal syndrome in the neonatal period in a newborn (see section “Use during pregnancy and breastfeeding”).
From the reproductive system: often – erectile dysfunction; infrequently – pain in the mammary glands, discomfort in the mammary glands, amenorrhea, galactorrhea, dysmenorrhea; rarely – menstrual irregularities, sexual dysfunction; frequency unknown – priapism, gynecomastia.
From the side of the organ of vision: often – oculogyric crisis, visual impairment, blurred vision.
General disorders: infrequently – hyperthermia, edema, gait disturbances; frequency unknown – sudden death, facial edema, hypothermia.
Interaction
Interaction
Concomitant use with drugs that prolong the QT interval (quinidine, disopyramide, amiodarone, dronedarone, sotalol, azithromycin, clarithromycin, erythromycin, levofloxacin, phenothiazine, methadone) is contraindicated.
Concomitant use of alcohol and haloperidol may enhance the effects of alcohol and lead to arterial hypotension.
Increases the severity of the inhibitory effect on the central nervous system of ethanol, tricyclic antidepressants, opioid analgesics, barbiturates and other hypnotic drugs, agents for general anesthesia.
Enhances the effect of peripheral m-anticholinergic drugs and most antihypertensive drugs (reduces the effect of guanethidine due to its displacement from alpha-adrenergic neurons and suppression of its uptake by these neurons).
Inhibits the metabolism of tricyclic antidepressants and monoamine oxidase inhibitors, thereby increasing (mutually) their sedative effect and toxicity.
When used simultaneously with bupropion, it reduces the epileptic threshold and increases the risk of grand mal seizures.
Reduces the effect of anticonvulsants (lowering the seizure threshold with haloperidol).
Weakens the vasoconstrictor effect of dopamine, phenylephrine, norepinephrine, ephedrine and epinephrine (blockade of alpha-adrenergic receptors by haloperidol can lead to a distortion of the action of epinephrine and a paradoxical decrease in blood pressure). Reduces the effect of antiparkinsonian drugs (antagonistic effect on dopaminergic structures of the central nervous system).
Changes (may increase or decrease) the effect of anticoagulants.
Reduces the effect of bromocriptine (dose adjustment may be required).
When used with methyldopa, it increases the risk of developing mental disorders (including disorientation in space, slowdown and difficulty in thinking processes).
Amphetamines reduce the antipsychotic effect of haloperidol, which, in turn, reduces their psychostimulant effect (blockade of alpha-adrenergic receptors by haloperidol).
M-anticholinergic drugs, first generation H1-histamine receptor blockers and antidyskinetic drugs may enhance the m-anticholinergic effect of haloperidol and reduce its antipsychotic effect (dose adjustment may be required).
Long-term use of carbamazepine, barbiturates and other inducers of microsomal oxidation reduces the concentration of haloperidol in plasma.
When used simultaneously with lithium preparations (especially in high doses), the development of encephalopathy (can cause irreversible neurointoxication) and increased extrapyramidal symptoms is possible.
When taken simultaneously with fluoxetine, the risk of developing side effects from the central nervous system, especially extrapyramidal reactions, increases.
When used simultaneously with drugs that cause extrapyramidal reactions, it increases the frequency and severity of extrapyramidal disorders.
Drinking strong tea or coffee (especially in large quantities) reduces the effect of haloperidol.
Overdose
Overdose
Due to the wide therapeutic index, intoxication usually develops only in cases of severe overdose.
Overdose symptoms:
severe extrapyramidal disorders: acute dyskinetic or dystonic symptoms, glossopharyngeal syndrome, gaze convulsions, spasms of the larynx, pharynx;
drowsiness, sometimes coma, agitation and confusion with delirium;
less often – epileptic seizures;
hyperthermia or hypothermia;
cardiovascular system: decreased or increased blood pressure, tachycardia or bradycardia, ECG changes such as prolongation of the PQ and QT intervals, ventricular flutter and fibrillation, cardiovascular failure;
rarely – m-anticholinergic effects: blurred vision, an attack of increased intraocular pressure, intestinal paresis, urinary retention;
rarely – respiratory complications: cyanosis, respiratory depression, respiratory failure, aspiration, pneumonia.
Treatment. There is no specific antidote. Treatment is symptomatic and supportive, following the general principles of overdose treatment, and the following circumstances must be taken into account.
Attempts to induce vomiting may be complicated by the antiemetic effect of antipsychotic drugs. Due to rapid absorption, gastric lavage is recommended only in cases of early diagnosis of overdose. Forced diuresis and dialysis are ineffective.
Analeptics are contraindicated, since with the use of haloperidol, due to a decrease in the threshold of convulsive readiness, there is a tendency to develop epileptic seizures. If severe extrapyramidal symptoms develop, antiparkinsonian drugs should be used, for example, intravenous biperiden; Antiparkinsonian medications may be required for several weeks. Patients in a coma are intubated. Spasm of the pharyngeal muscles may make intubation difficult; in this case, it is possible to use short-acting muscle relaxants.
In patients with symptoms of intoxication, ECG indicators and basic functional indicators of the body should be constantly monitored until they return to normal.
In case of arterial hypotension, due to increased paradoxical effects, adrenaline drugs (epinephrine (adrenaline)), which affect blood circulation, should not be used; drugs such as norepinephrine (eg, continuous drip infusions of norepinephrine (norepinephrine)) or angiotensinamide should be used instead. Beta-agonists should be avoided because they cause increased vasodilation.
Hypothermia is treated with slow rewarming. Infusion solutions for use in patients with hypothermia should be warmed.
For severe fever, antipyretics should be used and, if necessary, ice baths.
M-anticholinergic symptoms can be relieved by the use of physostigmine (1-2 mg IV) (repeat if necessary); use in standard daily practice is not recommended due to the severe side effects of this drug.
Anticonvulsants are used to treat recurrent epileptiform seizures, provided mechanical ventilation is possible due to the risk of respiratory depression.
Storage conditions
Storage conditions
Store at a temperature not exceeding 25°C.
Keep out of the reach of children!
Shelf life
Shelf life
5 years.
After opening the bottle, the drug is suitable for use for 6 months.
Do not use after expiration date.
Manufacturer
Manufacturer
Merkle GmbH, Germany
Additional information
| Shelf life | 5 years. After opening the bottle the drug is suitable for use within 6 months. Do not use after the expiration date. |
|---|---|
| Conditions of storage | Store at a temperature not exceeding 25°C. Keep out of reach of children! |
| Manufacturer | Merkle GmbH, Germany |
| Medication form | oral drops |
| Brand | Merkle GmbH |
Other forms…
Related products
Buy Haloperidol-Ratiofarm, drops 2 mg/ml 30 ml with delivery to USA, UK, Europe and over 120 other countries.