No products in the cart.
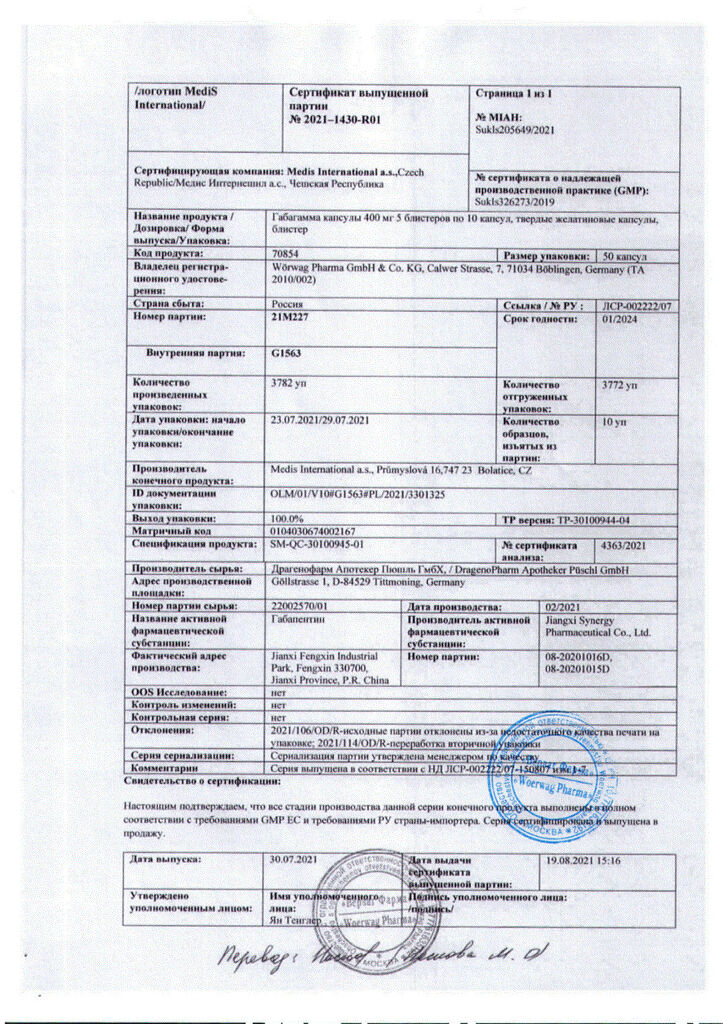
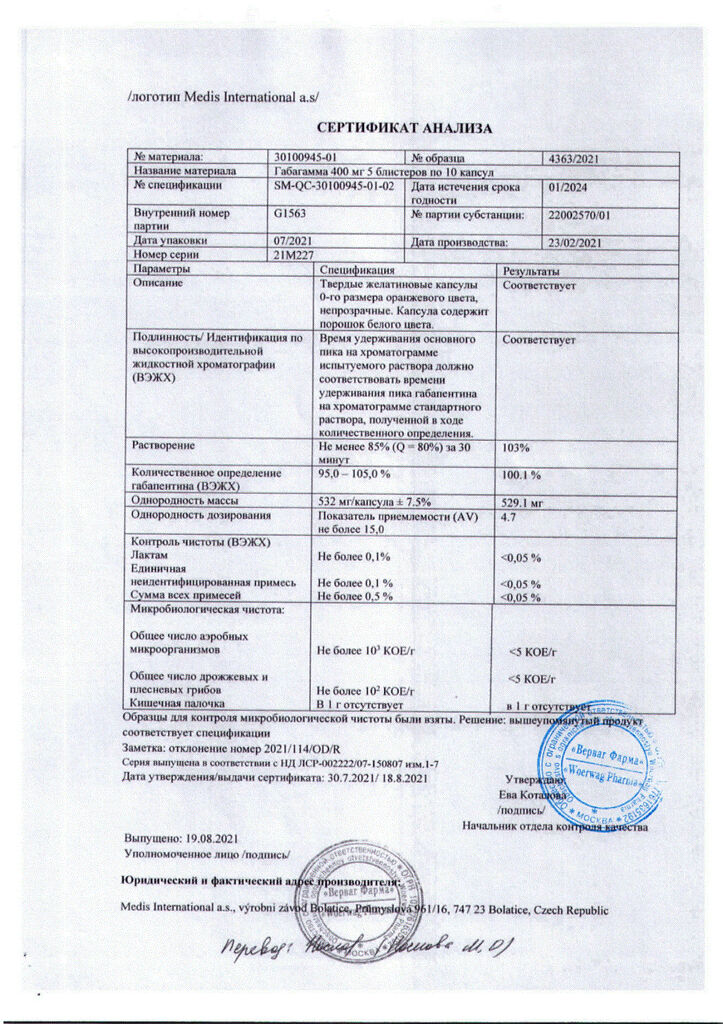
Gabagamma, 400 mg capsules, 50 pcs.
€1.00
Out of stock
(E-mail when Stock is available)
Description
Seizures, Epilepsy
- in complex therapy of partial seizures with or without secondary generalization in adults and children over 12 years old;
- pain syndrome in diabetic neuropathy, postherpetic neuralgia in adults
.
Active ingredient
Active ingredient
Composition
Composition
Solid gelatin capsules, #0, orange in color; the contents of the capsules – powder of white color.
Associates:
Lactose,
Corn starch,
Talc,
Gelatin,
How to take, the dosage
How to take, the dosage
The drug is taken orally, with or without food. If it is necessary to reduce the dose, discontinue the drug or replace it with an alternative remedy, this should be done gradually over at least 1 week.
Neuropathic pain in adults
The starting dose is 900 mg/day in 3 doses in equal doses; if necessary, the dose is gradually increased to a maximum of 3600 mg/day. Treatment may begin immediately with a dose of 900 mg/day (300 mg 3 times daily), or the dose may be increased gradually to 900 mg/day over the first 3 days, according to the following schedule:
Day 1: 300 mg of the drug 1 time/day;
Day 2: 300 mg 2 times/day;
Day 3: 300 mg 3 times/day.
Parietal seizures in adults and children from 12 years of age
The effective dose is 900 to 3,600 mg/day. Therapy may be started with a dose of 300 mg 3 times/day on the first day or increased gradually to 900 mg according to the scheme described above. Subsequently, the dose may be increased to 3,600 mg/day (divided into 3 equal doses). The maximum interval between doses in triplicate doses should not exceed 12 hours in order to avoid recurrence of seizures.
It is not necessary to monitor the plasma concentration of gabapentin. It may be used in combination with other anticonvulsants without regard to changes in its plasma concentrations or serum concentrations of other antiepileptic drugs (AEDs).
Dose selection in renal impairment
Patients with renal impairment are recommended to reduce the dose of gabapentin according to the table:
*- Prescribe 300 mg every other day.
Patients on hemodialysis who have not previously taken gabapentin are advised to prescribe the drug at a saturation dose of 300-400 mg and then use 200-300 mg every 4 hours of hemodialysis.
In debilitated patients, as well as in patients in severe general condition, with low body weight, and after organ transplantation, dose escalation should be done gradually using a dosage of 100 mg.
The use in elderly patients
There have been no systematic studies with gabapentin in patients 65 years and older. In a double-blind study, it was shown that patients with neuropathic pain aged 65 years or older had a slightly higher percentage of drowsiness, peripheral edema, and asthenia than younger individuals. Along with these observations, clinical studies in this age group do not indicate that the adverse event profile differs from that observed in younger patients.
Interaction
Interaction
A study of healthy volunteers (n=12) showed that when morphine was coadministered in controlled-release capsules of 20 mg/caps. 2 hours before gabapentin administration, there was a 44% increase in the average AUC of gabapentin compared to this value without morphine. Patients taking morphine and gabapentin together should be closely monitored due to the risk of developing CNS depression symptoms (drowsiness). If these symptoms occur, the dose of gabapentin or morphine should be reduced.
Interaction between gabapentin and phenobarbital, phenytoin, valproic acid or carbamazepine has not been observed. The equilibrium pharmacokinetics of gabapentin are the same in healthy subjects and patients with epilepsy receiving other anticonvulsants.
The concomitant use of gabapentin and oral contraceptives containing norethindrone and/or ethinylestradiol does not affect the pharmacokinetics of either component.
The bioavailability of gabapentin is reduced by 24% when taking gabapentin concomitantly with antacids containing aluminum and magnesium. It is recommended that gabapentin be taken approximately 2 h after taking antacids.
The renal excretion of gabapentin does not change when probenecid is taken.
When co-administered with cimetidine, there is a slight decrease in renal excretion of gabapentin that is not clinically significant.
Special Instructions
Special Instructions
Suicidal ideation, suicidal thoughts or worsening clinical picture
Suicidal thoughts and behavior have been reported in patients receiving antiepileptic drugs for multiple indications. A meta-analysis of randomized placebo-controlled trials of antiepileptic drugs also showed a small increase in the risk of suicidal thoughts and behavior. The mechanism of this risk is not known, and the data do not allow us to rule out the possibility of an increased risk with gabapentin.
Patients should be closely monitored for suicidal thoughts and behaviors. If these signs occur, appropriate treatment should be prescribed. Patients and caregivers should be advised to seek medical attention if they show signs of suicidal thoughts or behaviors.
Drug rash with eosinophilia and systemic symptoms (DRESS syndrome)
Severe, life-threatening, systemic hypersensitivity reactions such as drug rash with eosinophilia and systemic symptoms (DRESS syndrome) have been reported in patients taking anti-epileptic drugs, including gabapentin.
It should be noted that early manifestations of hypersensitivity, such as fever or enlarged lymph nodes (lymphoadenopathy), may appear even if a rash is not present. If such signs or symptoms appear, the patient should be evaluated immediately. Use of gabapentin should be discontinued unless an alternative cause for these symptoms can be identified.
If a patient treated with gabapentin develops acute pancreatitis, treatment with gabapentin should be stopped.
While no withdrawal syndrome has been observed with treatment with gabapentin, abrupt discontinuation of treatment is not recommended. Withdrawal of any anticonvulsants in patients with epilepsy may provoke epileptic status.
Treatment with gabapentin, as with other anticonvulsant medications, may increase the frequency of seizures or the appearance of new types of seizures in some patients. Monotherapy with gabapentin in patients who are resistant to anticonvulsant therapy has not been as successful as with other antiepileptic drugs.
Gabapentin is not effective in primary generalized seizures, such as absences, and may exacerbate these seizures in some patients. Gabapentin should be used with caution in patients with mixed seizures, including absences-epilepsy.
Patients 65 years and older
There have been no systematic studies with gabapentin in patients 65 years and older. In a double-blind study, it was shown that patients with neuropathic pain aged 65 years or older had a slightly higher percentage of drowsiness, peripheral edema, and asthenia than younger individuals. Along with these observations, clinical studies in this age group do not indicate that the adverse event profile differs from that observed in younger patients.
Children 12 years and older
The effects on learning, intelligence, and development in children and adolescents of long-term (more than 36 weeks) therapy with gabapentin have not been well studied. Therefore, the benefits of long-term therapy must be evaluated against the potential risks of such treatment.
Laboratory indicators
False positive results may be obtained when semi-quantitative determination of total protein in urine using test strips. If this is the case, it is recommended that a positive result be confirmed with other analytical methods such as Buret, turbidimetric, or dye-binding methods, or that alternative methods be used from the start.
The drug Gabagamma® contains lactose. The drug is contraindicated in patients with hereditary galactose intolerance, lactase deficiency or glucose-galactose malabsorption.
Influence on driving and operating machinery
Gabapentin may have slight to moderate effect on the ability to drive and operate machinery. The drug acts on the CNS and may cause drowsiness, dizziness and other symptoms. If these adverse effects are expressed even to a mild degree, they may be potentially dangerous for driving vehicles or operating mechanisms. Symptoms may occur more frequently at the beginning of treatment and with increasing doses.
Contraindications
Contraindications
With caution: renal failure, psychotic disorders.
Side effects
Side effects
The frequency of adverse reactions reported during clinical trials that were conducted in patients with epilepsy (in complex therapy and in gabapentin monotherapy) and in patients with neuropathic pain is distributed in the following order: very frequently (more than 10% of cases), frequently (1%-10% of cases), infrequently (01%-1% of cases), infrequent (0.01%-0.1% of cases), very rare (less than 0.01% of cases, including reports of isolated side effects). Where different frequencies of adverse effects were noted, the highest frequency is indicated. The frequency of adverse reactions noted in post-marketing observations cannot be estimated on the basis of available data. In the data below, their frequency is listed as “unknown”. In each group, adverse effects are presented in descending order of severity.
Infections and parasitic diseases: very common – viral diseases; common – pneumonia, respiratory diseases, urinary tract infections, infectious diseases, otitis media.
Hematopoietic system: common – leukopenia; unknown – thrombocytopenia.
The immune system: rare – allergic reactions (including urticarial rash); unknown – drug rash with eosinophilia and systemic symptoms (DRESS syndrome).
Metabolism and nutrition: often – anorexia, increased appetite.
Psychiatric disorders: often – hostility, confusion and emotional lability, depression, anxiety, increased nervous excitability, thought disorders; unknown – hallucinations.
Nervous system disorders: very frequently – somnolence, dizziness, ataxia; frequently – convulsions, hyperkinesias, dysarthria, amnesia, tremor, sensory disorders, such as paresthesia, decreased sensitivity, movement coordination disorders, nystagmus, increase, decrease or absence of reflexes; rarely – hypokinesia; unknown – other disorders of motor functions (including dyskinesia, dystonia).
Visually: often – visual disturbances such as decreased visual acuity, double vision.
Hearing organ and labyrinth disorders: frequent – dizziness; unknown – tinnitus.
Cardiovascular system: often – arterial hypertension, vasodilation; rarely – palpitations.
Respiratory system: often – dyspnea, bronchitis, pharyngitis, cough, rhinitis.
The digestive system: frequent – vomiting, nausea, dental anomalies, gingivitis, diarrhea, abdominal pain, dipepsia, constipation, dry mouth and throat, flatulence; unknown – pancreatitis, hepatitis, jaundice.
Skin and subcutaneous tissue disorders: often – facial edema, hemorrhagic rash most commonly described as bruising due to physical trauma, rash, itching, acne; unknown – Stevens-Johnson syndrome, angioneurotic edema, erythema multiforme, alopecia.
Muscular system and connective tissue: often – joint pain, muscle pain, back pain, twitching; unknown – myoclonus.
Renal and urinary tract disorders: unknown – acute renal failure, urinary incontinence.
Gender and mammary glands: often – impotence; unknown – breast hypertrophy, gynecomastia.
General disorders and disorders at the site of administration: very often – fatigue, fever; often – peripheral edema, gait disturbances, asthenia, pain, malaise, flu-like syndrome; rarely – generalized edema; unknown – withdrawal syndrome (mostly anxiety, insomnia, nausea, pain, sweating), chest pain. Cases of sudden unexpected death have been reported where a causal link to gabapetpin treatment has not been established.
Laboratory and instrumental studies: frequently – leukopenia, weight gain; rarely – increased liver enzymes (ACT, ALT) and bilirubin levels; unknown – fluctuations in blood glucose levels in diabetic patients.
Injuries, intoxications and complications of manipulations: often – accidental trauma, fracture, abrasions.
In treatment with gabapentin, there are reports of cases of acute pancreatitis. A causal relationship to gabapentin ingestion has not been established.
There have been reported cases of myopathy with increased creatine kinase levels in terminally renal patients on hemodialysis.
Respiratory tract infections, otitis media, bronchitis, and seizures in children have only been reported in clinical trials. In addition, aggressive behavior and hyperkinesias have been reported in children in most clinical studies.
Overdose
Overdose
Symptoms: dizziness, diplopia, speech disorders, drowsiness, lethargy, diarrhea and increased severity of other side effects.
Treatment: gastric lavage, administration of activated charcoal, symptomatic therapy. Hemodialysis may be indicated in patients with severe renal failure.
Similarities
Similarities
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | The drug should be kept out of reach of children at a temperature not exceeding 25 ° C. |
| Manufacturer | Artesan Pharma GmbH & Co. KG, Germany |
| Medication form | capsules |
| Brand | Artesan Pharma GmbH & Co. KG |
Other forms…
Related products
Buy Gabagamma, 400 mg capsules, 50 pcs. with delivery to USA, UK, Europe and over 120 other countries.