No products in the cart.
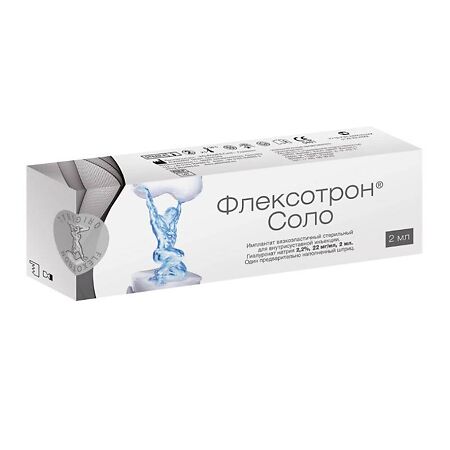
Flexotron Solo 2.2%, 22 mg/ml 2 ml, syringe
€476.48 €397.07
Description
– osteoarthritis / osteoarthritis (OA) and other degenerative-dystrophic and post-traumatic lesions of the knee, hip and other synovial joints.
– restoration of synovial fluid properties in orthopedic joint surgery, as well as in persons with increased stress on damaged joints.
Indications
Indications
– osteoarthritis / osteoarthritis (OA) and other degenerative-dystrophic and post-traumatic lesions of the knee, hip and other synovial joints.
– restoration of the properties of synovial fluid during orthopedic joint surgery, as well as in persons with increased loads on damaged joints.
Special instructions
Special instructions
During the first 2 days after the procedure, it is recommended not to overload the joint, especially prolonged loading should be avoided. When obtaining aspiration fluid, appropriate studies should be carried out before viscosaplimentary therapy to exclude a bacterial etiology of arthritis.
The product does not affect a person’s ability to drive a vehicle or engage in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Precautions
Because septic arthritis is a serious side effect, all standard precautions for surgical procedures must be followed.
The product is intended for intra-articular administration. Intravascular or interstitial administration of the product should be avoided.
Due to the lack of clinical data on the use of hyaluronic acid in the treatment of children, pregnant or lactating women, avoid using the product in these groups of patients.
The product should be used before the expiration date indicated on the packaging.
In the first days after the injection, oral analgesics or anti-inflammatory medications may be helpful.
The use of the product together with quaternary ammonium compounds is prohibited.
Re-sterilization and reuse of the product is prohibited due to the risk of infection, cross-infection and/or product defect.
For one time use only. Do not use a syringe from an open and/or damaged sterile package. Do not use a syringe with an open or damaged sterile syringe cap.
If the sterility of the implant is compromised or the sterility of the implant is suspected, the product must be disposed of.
Composition
Composition
1 ml contains:
Sodium hyaluronate 2.2% / 22 mg
Sodium chloride 8,500 mg
Sodium hydrogen phosphate dihydrate
Na2HPO4 * 2H2O 0.563 mg
Sodium Dihydrogen Phosphate Dihydrate
NaH2PO4 * 2H2O 0.045 mg
Water for injection up to 1 ml volume
Contraindications
Contraindications
The product should not be used to treat patients:
– having hypersensitivity to one of the components of the product;
– suffering from septic arthritis;
– those suffering from skin infections or dermatological diseases in the injection area;
– taking anticoagulants, such as, for example, Phenoprocoumon or Warfarin.
Side Effects
Side Effects
The use of intra-articular forms of hyaluronic acid for degenerative-dystrophic and post-traumatic joint lesions is a well-studied method with an established safety profile, used for several decades.
In extremely rare cases, local temporary inflammatory symptoms may occur (pain, fever, redness and swelling, increased exudate in the joint cavity). After an intra-articular injection, reversible local reactions may occur, such as short-term limitation of mobility (stiffness), a feeling of discomfort or heaviness in the joint, and hematomas. The manifestation of these symptoms can be reduced by applying ice to the injection site for 5-10 minutes.
There are reports of isolated cases of allergic reactions (for example, itching, rash, urticaria) and anaphylactic reactions, septic arthritis, interstitial bleeding or hemorrhage into the joint cavity, tendonitis, phlebitis, paresthesia, dizziness, headaches, muscle spasms, fever, general malaise, peripheral edema with intra-articular administration hyaluronic acid solutions.
If local or general symptoms appear, you should consult your doctor.
Interaction
Interaction
Implants of the FLEXOTRON® line have successfully passed preclinical tests and confirmed compatibility with biological tissues, cells and body fluids with which they come into contact in the implanted state. Disinfectants containing quaternary ammonium salts should not be used, since hyaluronic acid precipitates in the presence of these substances. There is currently no information about incompatibility with other drugs, substances and intra-articular injection products, however, a medical professional should carefully read the information in the instructions for these drugs/substances/products and use with caution.
Specifications
Specifications
Sterility is sterile; Sterilized by autoclaving according to EN ISO 17665-1 with Sterility Assurance Level (SAL) 10-6.
Type of pure homogeneous solution (gel)
No smell
Color colorless, transparent
Implant viscosity at zero shear rate: – Solo > 50,000 mPa•s, implant pH 6.8 – 7.4
Molecular weight 0.8 – 2.5 million Yes
Removable implant volume ≥ 2 ml
Osmolality 270 – 400 mOsmol/kg
Bacterial endotoxins <0.5 IU/ml
Mechanical impurities in the implant:
> 10 µm
> 25 µm subvisible particles
≤ 25,000 pcs/ml
≤ 5,000 pcs/ml
Recommended needle sizes (not part of this medical device) 21G, single use, sterile
Prescribing
Prescribing
Viscoelastic prosthetics of synovial fluid in patients with degenerative-dystrophic and post-traumatic joint lesions, as well as in persons with increased loads on damaged joints.
Complete set of goods
Complete set of goods
Each FLEXOTRON® version is supplied to the end user consisting of the following components:
– implant (liquid mixture of components, see table above) in a pre-filled syringe in a blister pack;
– 3 implantation stickers;
– instructions for use.
Action
Action
Hyaluronic acid is a poly-(2-acetamido-2-deoxy-D-gluco)-D-glucuronoglycan, that is, a polymer consisting of D-glucuronic acid and N-acetyl-D-glucosamine residues connected alternately by β-1,4- and β-1,3-glycosidic bonds. Thanks to this three-dimensional polymer structure, large negatively charged aggregates are formed that retain water. These units are responsible for the moisture content, firmness and elasticity of cartilage tissue (its resistance to compression).
Intra-articular enrichment of synovial fluid with injections of sodium hyaluronate helps to improve or restore the viscoelastic properties of natural synovial fluid. Sodium hyaluronate is responsible for the viscoelastic properties of synovial fluid, thus viscoelastic prosthetics makes it possible to compensate for the deficiency of hyaluronic acid in the synovial fluid or a decrease in its viscosity, soften external loads on the joint, provide lubrication, restore elasticity and viscosity, shock absorption, moisturizing and enveloping the articular surfaces, covering cartilage and receptors with a lubricating protective layer synovia. This helps increase range of motion and provides mechanical protection for the tissues of the joint cavity, which in turn can improve the course of osteorathrosis/osteoarthritis and other degenerative-dystrophic and post-traumatic pathologies of the joints.
Based on clinical information about the characteristics of biodegradation and the duration of the therapeutic effects of intra-articular injection of hyaluronic acid, it is assumed that the biodegradation of each version of the FLEXOTRON® viscoelastic implant occurs within a period of 12 to 24 weeks. The clinical effect of treatment lasts for at least 6 months.
Storage conditions
Storage conditions
from 2 °C to 25 °C in original packaging,
at relative humidity 30-60%;
protect from light;
do not freeze;
Avoid impacts and sudden shaking.
Keep out of the reach of children.
This medical device is transported by all types of transport in covered vehicles in accordance with the requirements, rules and regulations for the transportation of goods applicable to each type of transport.
Shelf life
Shelf life
The shelf life of FLEXOTRON® is limited to 3.5 years. The expiration date is indicated on the packaging and is valid subject to the conditions of transportation and storage. Do not use after expiration date.
Manufacturer
Manufacturer
CyVision Biotech Inc., Taiwan
Additional information
| Shelf life | The shelf life of FLEXOTRON® is limited to 3.5 years. The expiration date is indicated on the package and is valid under transportation and storage conditions. Do not use after the expiration date. |
|---|---|
| Conditions of storage | from 2°C to 25°C in the original package, in 30-60% relative humidity; Shield from light; No freezing; Avoid shock or violent shaking. Store out of the reach of children. This medical product is transported by all modes of transportation in covered vehicles in accordance with the requirements, rules, and regulations for each mode of transportation. |
| Manufacturer | Albomed GmbH, Germany |
| Medication form | implant |
| Brand | Albomed GmbH |
Related products
Buy Flexotron Solo 2.2%, 22 mg/ml 2 ml, syringe with delivery to USA, UK, Europe and over 120 other countries.