No products in the cart.
Favirox, 500 mg 21 pcs
€72.87 €64.40
Description
Herpes zoster (infection caused by VZV):
- to treat herpes zoster, including ophthalmoherpes in immunocompetent patients;
- to treat herpes zoster in immunocompromised patients.
Genital herpes (infection caused by HSV):
- treatment of first episode and recurrence of genital herpes in immunocompromised patients;
- treatment of recurrence of genital herpes in immunocompromised patients;
- to prevent exacerbations of genital herpes (suppressive therapy) in immunocompromised and immunocompromised patients.
Labial herpes (infection caused by HSV):
- treatment of recurrent labial herpes in immunocompromised patients;
- treatment of recurrent orolabial herpes in immunocompromised patients.
Active ingredient
Active ingredient
Composition
Composition
Each film-coated tablet, 500 mg contains:
the active ingredient:
famcyclovir – 500.00 mg;
excipients:
Pregelatinized starch – 74.80 mg,
microcrystalline cellulose – 44.00 mg,
croscarmellose sodium – 40.80 mg,
sodium lauryl sulfate – 6.80 mg,
silica colloidal anhydrous silica – 6.80 mg,
stearic acid – 6.80 mg;
film coating:
Opadray white OY-S-28924 (hypromellose-5cP – 7.48 mg, titanium dioxide – 4.08 mg, hypromellose-15cP – 2.48 mg, macrogol-4000 – 1.48 mg, macrogol-6000 – 1.48 mg) – 17.00 mg.
How to take, the dosage
How to take, the dosage
The drug should be taken orally, regardless of meals, without chewing, with water. Treatment with the drug should be started as soon as possible, immediately after the appearance of the first symptoms of the disease (tingling, itching and burning).
Infections caused by VZV (herpes zoster), including ophthalmoherpes in immunocompetent patients:
The recommended dose is 500 mg 3 times daily for 7 days.
Infection caused by VZV (herpes zoster) in immunocompromised patients:
The recommended dose is 500 mg 3 times daily for 10 days.
Infection caused by HSV (labial or genital herpes) in immunocompromised patients:
– For a first episode of genital herpes, the recommended dose is 250 mg 3 times daily for 5 days;
– For relapses of genital herpes, 1000 mg 2 times daily for 1 day or 125 mg 2 times daily for 5 days or 500 mg once, followed by 3 doses of 250 mg every 12 hours.
– For relapses of labial herpes – 1500 mg once daily for 1 day or 750 mg twice daily for 1 day.
Infection caused by HSV (orolabial or genital herpes) in immunocompromised patients:
The recommended dose is 500 mg twice daily for 7 days.
To prevent exacerbations of genital herpes (suppressive therapy), 250 mg 2 times daily is used. The duration of therapy depends on the severity of the disease. Periodic evaluation of possible changes in the course of the disease after 12 months is recommended.
In HIV-infected patients, the effective dose is 500 mg 2 times a day.
Patients aged â¥65 years
In elderly patients with normal renal function, dosing adjustment of famcyclovir is not necessary.
Patients with impaired renal function
Patients with impaired renal function have decreased clearance of penciclovir.
Recommendations for dosing regimen adjustment in immunocompetent patients with impaired renal function based on creatinine clearance are presented in Table 1.
Recommendations for dosing regimen adjustment in immunocompromised patients with impaired renal function as a function of creatinine clearance are presented in Table 2.
Table 1. Dosing regimen correction in immunocompromised patients with impaired renal function
Infection caused by VZV (herpes zoster)
Dosing regimen
Creatinine clearance
Adjusted dosing regimen
500 mg 3 times daily for 7 days
⥠60
500 mg 3 times daily for 7 days
40-59
500 mg 2 times daily for 7 days
20-39/p>
500 mg once daily for 7 days
< 20
250 mg once daily for 7 days
Patients on hemodialysis or receiving a hemodialysis procedure
250 mg after each dialysis session for 7 days
/p>
Infection caused by HSV
< 20
250 mg once
Patients on hemodialysis or receiving a hemodialysis procedure
< 20
250 mg once daily for 7 days
Patients on hemodialysis or receiving a hemodialysis procedure
250 mg after each dialysis session for 7 days
Patients with renal impairment who are on hemodialysis or receiving a hemodialysis procedure
Because penciclovir plasma concentrations are reduced by 75% after a 4-hour hemodialysis procedure, famiclovir should be taken immediately after the hemodialysis procedure. See Tables 1 and 2 for a recommended dose adjustment regimen.
Patients with hepatic impairment
There is no need for dose adjustment in patients with mild to moderate hepatic impairment.
There is no experience of using the drug in patients with severe hepatic impairment.
Patients of the Negro race
The efficacy of a 1-day dosage of famcyclovir 1000 mg twice daily for the treatment of genital herpes relapse in immunocompetent patients of the Negro race was not greater than that of placebo. The clinical significance of drug dosing regimens for the treatment of both genital herpes relapses (within 2 or 5 days) and other infectious lesions caused by VZV and HSV is unknown.
Interaction
Interaction
Special Instructions
Special Instructions
Synopsis
Synopsis
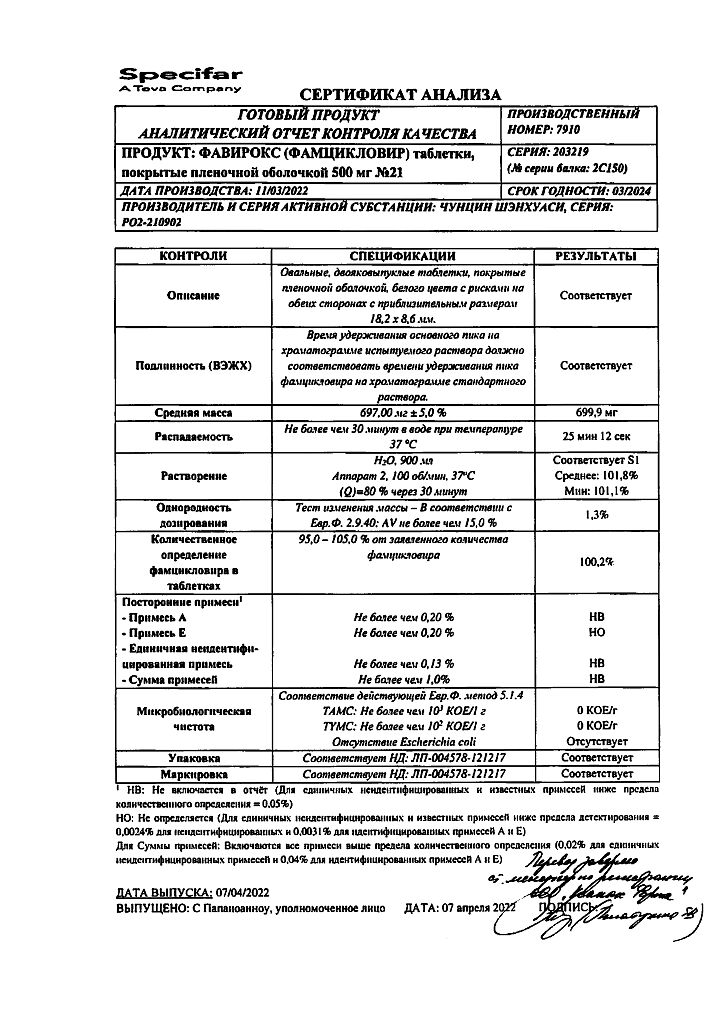
Filmed film-coated tablets, 500 mg:
Oval, biconvex, white film-coated tablets with marks on both sides of the tablet.
Contraindications
Contraindications
Hypersensitivity to famcyclovir or any of the ingredients of the drug. Hypersensitivity to penciclovir.
Children under 18 years of age due to lack of efficacy and safety data in patients in this age group.
Severe hepatic impairment due to lack of efficacy and safety data in this patient population.
Caution should be exercised when treating patients with impaired renal function, for whom dosing adjustments may be necessary.
Special precautions are not required in elderly patients and patients with mild to moderate hepatic impairment.
Side effects
Side effects
Overdose
Overdose
Pregnancy use
Pregnancy use
Additional information
| Shelf life | 2 years. |
|---|---|
| Conditions of storage | Store at a temperature not exceeding 25 ° C, in the original container. |
| Manufacturer | Specifar S.A., Greece |
| Medication form | pills |
| Brand | Specifar S.A. |
Other forms…
Related products
Buy Favirox, 500 mg 21 pcs with delivery to USA, UK, Europe and over 120 other countries.