No products in the cart.
Enbrel, lyophilizate 10 mg 4 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
A drug with anti-inflammatory action. Tumor necrosis factor alpha (TNF-α) inhibitor
Indications
Indications
Juvenile idiopathic polyarthritis
– treatment of active juvenile idiopathic polyarthritis in children and adolescents aged 2-17 years who have had insufficient effectiveness or intolerance to methotrexate.
Psoriasis
– treatment of children aged 6 years and older with severe chronic psoriasis who have experienced intolerance or insufficient response to other systemic or phototherapy.
Pharmacological effect
Pharmacological effect
A drug with an anti-inflammatory effect. Tumor necrosis factor alpha (TNF-α) inhibitor
Special instructions
Special instructions
After stopping Enbrel, symptoms may recur.
Infections
Patients should be monitored for infections before starting Enbrel, during treatment, and after completion of therapy, taking into account the average half-life of etanercept of approximately 70 hours (7-300 hours).
Sepsis, tuberculosis, parasitic infections (including protozoa), and severe infections, including opportunistic infections, including invasive fungal infections, listeriosis, and legionellosis, have been reported with use of Enbrel. Incorrect diagnosis of these infections, especially fungal and other opportunistic infections, led to delayed administration of the necessary treatment and, in some cases, death.
When examining patients, one must take into account the possibility of them developing opportunistic infections, for example, endemic mycoses. Patients who develop new infections during treatment with Enbrel should be closely monitored. Enbrel should be discontinued if the patient develops a severe infection. Enbrel should be used with extreme caution in patients with a history of frequent or chronic infections or who have an underlying medical condition, such as advanced or poorly controlled diabetes, that may precipitate the development of infections.
The safety and effectiveness of Enbrel in patients with chronic infections has not been evaluated.
Tuberculosis
Cases of active tuberculosis, including miliary tuberculosis and extrapulmonary tuberculosis, have been reported during therapy with Enbrel.
Before starting therapy, all patients should be screened for active or latent tuberculosis. The examination should include a detailed examination of the patient’s history of tuberculosis or previous contact with tuberculosis patients and information about previous or current immunosuppressive therapy. All patients should undergo appropriate screening procedures (according to local requirements), namely tuberculin skin test and chest radiography. The possibility of a false-negative tuberculin test should be considered, especially in patients in serious condition or patients with compromised immunity.
Enbrel should not be used if the patient has active tuberculosis. The diagnosis of inactive tuberculosis requires the initiation of standard anti-tuberculosis therapy before initiating Enbrel therapy. In this case, the benefit-risk ratio of treatment with Enbrel should be carefully analyzed.
All patients should be informed of the need to consult a doctor if complaints or symptoms characteristic of tuberculosis (for example, persistent cough, weight loss, low-grade fever) appear during or after treatment with Enbrel.
Activation of hepatitis B virus
Cases of hepatitis B virus activation have been reported in carrier patients who received TNF inhibitors, including Enbrel. Before using Enbrel in patients at high risk of hepatitis B, an appropriate diagnostic search must be performed. Particular caution should be exercised when using Enbrel in patients who are carriers of the hepatitis B virus. If they develop symptoms of this disease, the possibility of specific therapy should be discussed.
Exacerbation of hepatitis C
Cases of exacerbation of hepatitis C have been reported in patients receiving therapy with Enbrel. Caution should be exercised when using Enbrel in patients with a history of hepatitis C.
Allergic reactions
Allergic reactions often accompany taking Enbrel. If any severe allergic or anaphylactic reactions occur, stop taking Enbrel immediately and initiate appropriate treatment.
Immunosuppression
When treated with TNF inhibitors, including Enbrel, there is a possibility of suppressing the human body’s defense mechanisms against infections and malignancies, since TNF is involved in inflammation and modulates the cellular immune response. However, in adult patients with rheumatoid arthritis during treatment with Enbrel, there were no cases of inhibition of delayed hypersensitivity reactions, a drop in immunoglobulin levels, or changes in the population of effector cells.
In rare cases, children with juvenile idiopathic polyarthritis developed chickenpox and symptoms of aseptic meningitis, which resolved without complications. Patients who have been in contact with patients with chickenpox should temporarily stop taking Enbrel and receive prophylactic treatment with immunoglobulin against the Varicella zoster virus.
The effectiveness and safety of treatment with Enbrel in patients with immunosuppression have not been studied.
Malignant and lymphoproliferative diseases
During post-marketing experience (see Adverse Reactions section), reports of various malignancies (including breast and lung carcinoma and lymphoma) have been received.
Lymphoma was diagnosed more often in patients taking TNF inhibitors than in patients who were not taking them. On the other hand, these cases were rare, and the follow-up period for patients in the placebo group was shorter than for patients receiving treatment with TNF inhibitors. In addition, there is a high risk of lymphoma and leukemia in patients with rheumatoid arthritis, which is a long-term disease characterized by active inflammation, which itself complicates risk assessment. However, according to current data, a possible risk of developing lymphomas, leukemia or other malignant neoplasms cannot be excluded in patients receiving TNF inhibitors.
There are reports of the development of malignant neoplasms, some of which were fatal, in children and adolescents treated with TNF inhibitors, including Enbrel. Approximately half of these cases developed lymphoma. Other cases involved a variety of malignancies, including rare variants associated with immunosuppression. When using the drug, it is necessary to take into account the risk of developing malignant neoplasms in children and adolescents.
Skin cancer
Melanoma and non-melanoma skin cancer (NMSC) have been reported in patients treated with TNF inhibitors, including Enbrel. Most often, RCNM is diagnosed in patients with psoriasis. There are reports of the development of Merkel cell carcinoma. For all patients at risk, periodic examination of the skin is recommended.
Formation of autoimmune antibodies
Treatment with Enbrel may be accompanied by the formation of autoimmune antibodies (see section “Side effects”). These antibodies are not neutralizing and usually disappear quickly. There was no correlation between the formation of antibodies and the clinical effectiveness of the drug, as well as the incidence of adverse reactions. Isolated cases of the formation of additional autoantibodies in combination with a lupus-like syndrome or a rash similar to subacute lupus erythematosus or discoid lupus erythematosus (data from clinical examination and biopsy) were observed in patients, including patients with rheumatoid arthritis with positive rheumatoid factor.
Hematological reactions
Rare cases of pancytopenia and very rare cases of aplastic anemia, including death, have been reported in patients receiving Enbrel. Caution should be exercised when using Enbrel in patients with a history of blood diseases. All patients and their relatives/caregivers should be aware that if a patient develops signs and symptoms consistent with an infection or hematologic disorder (eg, prolonged fever, sore throat, bruising, bleeding, pallor) while taking Eibrel, they should seek immediate medical attention. In such patients, prompt evaluation, including a complete blood count, is recommended. If the diagnosis of a hematological disease is confirmed, treatment with Enbrel should be discontinued.
CNS damage
Although the use of Enbrel in patients with multiple sclerosis has not been studied, studies of other TNF inhibitors in this concomitant disease have shown the possibility of its exacerbation.
Before initiating therapy with Enbrel, it is recommended to carefully evaluate the risk/benefit ratio and neurological status in patients with a previous exacerbation of a demyelinating disease and in patients at increased risk of developing a demyelinating disease.
Combination therapy
The combination of Enbrel and methotrexate did not produce unexpected results in a safety study. Long-term study of this indicator is ongoing. Safety data for Enbrel given with methotrexate were similar to those reported for use of Enbrel and methotrexate alone. Long-term safety when taking Enbrel with other disease-modifying anti-inflammatory drugs has not been studied.
Enbrel® has not been studied in combination with other systemic therapy or phototherapy for psoriasis.
Wegener’s granulomatosis
The incidence of various types of extracutaneous malignancies was significantly higher in patients with Wegener’s granulomatosis treated with Enbrel than in the control group.
Therefore, Enbrel is not recommended for the treatment of patients with Wegener’s granulomatosis.
Hypoglycemia in patients with diabetes mellitus
Cases of hypoglycemia have been reported during therapy with Enbrel in patients taking antidiabetic drugs, which required dose adjustment.
Impact on the ability to drive a car and use complex equipment
The effect on the ability to drive a car and use complex equipment when using Enbrel has not been studied. In this regard, you should drive a car or use complex equipment with caution.
Active ingredient
Active ingredient
Etanercept
Composition
Composition
1 fl. etanercept 10 mg
Excipients:
mannitol 40 mg,
sucrose 10 mg,
trometamol (as a mixture of trometamol and trometamol hydrochloride to achieve a pH value of 7.4) 1.2 mg.
Contraindications
Contraindications
– hypersensitivity to etanercept or any other component of the drug;
– sepsis or risk of sepsis;
– active infection, including chronic or localized infections;
– pregnancy and breastfeeding.
The effectiveness and safety of Enbrel for the treatment of juvenile idiopathic polyarthritis in children under 2 years of age have not been studied.
The effectiveness and safety of Enbrel for the treatment of psoriasis in children under 6 years of age have not been studied.
With caution
Demyelinating diseases, congestive heart failure (CHF), immunodeficiency conditions, diseases predisposing to the development or activation of infections (diabetes mellitus, hepatitis), moderate and severe alcoholic hepatitis, hepatitis C, blood dyscrasia, nervous diseases (multiple sclerosis, optic neuritis, transverse myelitis).
Side Effects
Side Effects
Adverse reactions depending on the frequency of occurrence were grouped as follows: very often (≥1/10); often (≥1/100, <1/10); uncommon (≥ 1/1000, < 1/100); rare (≥1/10,000, <1/1000); very rare (< 1/10000), isolated cases (frequency cannot be determined).
Infectious and parasitic diseases: very often – infections (including upper respiratory tract infections, bronchitis, cystitis, skin infections); uncommon – serious infections (including pneumonia, cellulitis, septic arthritis, sepsis and parasitic infections); rarely – tuberculosis, opportunistic infections (including invasive fungal, protozoal, bacterial and atypical mycobacterial infections and diseases caused by Legionella); isolated cases are infections caused by Listeria.
Benign, malignant and unspecified neoplasms (including cysts and polyps): uncommon – skin cancer not related to melanoma (SCNM); rarely – lymphoma, melanoma; isolated cases – leukemia, Merkel carcinoma.
Disorders of the blood and lymphatic system: infrequently – thrombocytopenia; rarely – anemia, leukopenia, neutroemia, pancytopenia; very rarely – anlastic anemia.
Immune system disorders: often – allergic reactions (see subsection “Skin and subcutaneous tissue”), formation of autoantibodies; uncommon – systemic vasculitis (including ANCA-associated vasculitis); rarely – serious allergic/anaphylactic reactions (including angioedema, bronchospasm), sarcoidosis; isolated cases – macrophage activation syndrome.
Nervous system disorders: rarely – convulsions, demyelination phenomena in the central nervous system, similar to those observed in multiple sclerosis or local demyelination conditions, such as optic neuritis and transverse myelitis (see section “Special Instructions”); very rarely – peripheral demyelinating diseases (including Guillain-Barré syndrome, chronic inflammatory demyelinating polyneuropathy, demyelinating polyneuropathy, multifocal motor neuropathy).
Violations of the organ of vision: infrequently – uveitis.
Disorders of the respiratory system, chest and mediastinal organs: infrequently – interstitial pulmonary diseases (including pneumonitis and pulmonary fibrosis).
Disorders of the liver and biliary tract: rarely – increased activity of liver enzymes, autoimmune hepatitis.
Disorders of the skin and subcutaneous tissues: often – itchy skin; uncommon – angioedema, urticaria, rash, psoriasis-like rash, psoriasis (including disease onset or worsening and pustular lesions, mainly of the soles and palms); rarely – cutaneous forms of vasculitis, Stevens-Johnson syndrome, erythema multiforme; very rarely – toxic epidermal necrolysis.
Disorders of the musculoskeletal system and connective tissue: rarely – cutaneous manifestations of subacute lupus erythematosus, discoid lupus erythematosus, lupus-like syndrome.
General disorders and disorders at the injection site: very often – local reactions after injections (including bleeding, formation of subcutaneous hematoma, erythema, itching, pain, swelling); often – fever.
Disorders of the cardiovascular system: rarely – worsening of congestive heart failure (CHF) (see section “Special instructions”).
Adverse reactions in children
The frequency and types of adverse reactions in children with juvenile idiopathic polyarthritis were similar to those observed in adult patients with rheumatoid arthritis. Infections observed in clinical studies in children with juvenile idiopathic polyarthritis aged 2 to 18 years were mild to moderate in severity and consistent with those commonly encountered in outpatients. Reports of severe adverse events included varicella with symptoms of aseptic meningitis that resolved without complications (see Precautions section), appendicitis, gastroenteritis, depression/personality disorders, skin ulcers, esophagitis/gastritis, aseptic shock due to group A streptococci, type 1 diabetes mellitus, and soft tissue and surgical wound infections.
There have been 4 reports of macrophage activation syndrome in these patients. Cases of inflammatory bowel disease have been reported in patients with juvenile idiopathic polyarthritis receiving Enbrel therapy in the post-marketing period, including cases with recurrent bowel disease. A clear cause-and-effect relationship has not been established, since cases of inflammatory bowel disease also occurred in patients with juvenile idiopathic polyarthritis who did not receive treatment.
The frequency and types of adverse reactions in children with psoriasis were similar to those observed in adult patients.
Interaction
Interaction
Anakinra
During combination therapy with Enbrel and anakinra, there was a significant increase in the incidence of serious infections and neutropenia compared with patients treated with Enbrel or anakinra alone. Concomitant use of Enbrel and anakinra has not shown clinical benefit and is therefore not recommended.
Abatacept
Concomitant use of abatacept and Enbrel was associated with an increased incidence of serious adverse events. This drug combination has not demonstrated clinical benefit and is therefore not recommended.
Sulfasalazine
Significant reductions in white blood cell counts have been reported in patients treated with Enbrel while receiving sulfasalazine compared to patients treated with Enbrel or sulfasalazine alone.
Lack of interaction
No adverse interactions were observed when Enbrel was co-administered with glucocorticosteroids, salicylates (except sulfasalazine), NSAIDs, analgesics or methotrexate.
Methotrexate
Methotrexate has no effect on the pharmacokinetics of etanercept. The effect of Enbrel on the pharmacokinetics of methotrexate in humans has not been studied.
Digoxin
No clinically significant mutual influence was found on the pharmacokinetics of etanercept.
Warfarin
No clinically significant mutual effects on the pharmacokinetics of etanercept were found.
Vaccination
Live vaccines should not be administered during treatment with Enbrel. There is no evidence of secondary transmission of infection through live vaccine in patients receiving Enbrel. It is recommended that, if possible, patients with juvenile idiopathic polyarthritis receive all required vaccinations in accordance with the current national immunization schedule before starting treatment with Enbrel.
Overdose
Overdose
If you take an overdose of Enbrel, tell your doctor or pharmacist immediately. Save the Enbrel carton, even if it is empty.
When treating patients with rheumatoid arthritis, no cases of exceeding the toxic dose limit have been reported.
The highest dose administered intravenously was 32 mg/m2, followed by subcutaneous administration of 16 mg/m2 twice weekly.
One patient with rheumatoid arthritis erroneously self-administered 62 mg of Enbrel subcutaneously twice weekly for 3 weeks without adverse effects. A specific antidote for Enbrel is unknown.
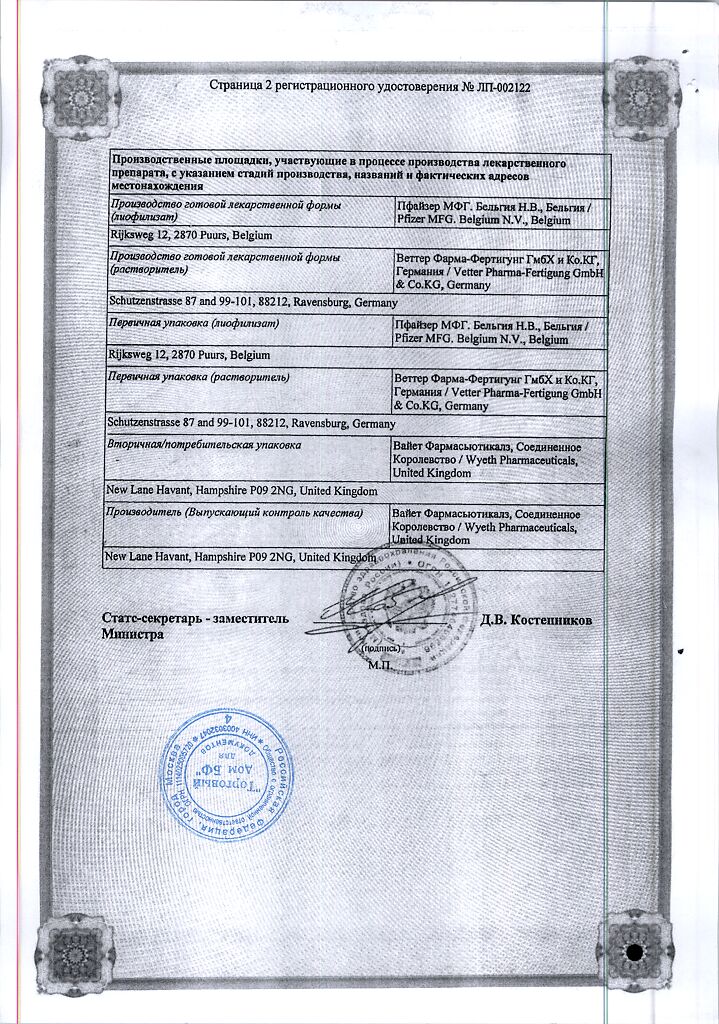
Manufacturer
Manufacturer
USA
Additional information
| Manufacturer | Pfizer, Puerto Rico |
|---|---|
| Medication form | lyophilizate |
| Brand | Pfizer |
Related products
Buy Enbrel, lyophilizate 10 mg 4 pcs with delivery to USA, UK, Europe and over 120 other countries.