No products in the cart.
Duspatalin, capsules 200 mg 30 pcs
€16.85 €15.83
Description
Pharmacodynamics
A myotropic antispasmodic. It has a direct effect on the smooth muscles of the gastrointestinal tract. It relieves spasm without affecting normal intestinal peristalsis.
It has no anticholinergic effect.
Pharmacokinetics
Intake
Mebeverin is quickly and completely absorbed after oral administration. The modified-release dosage form allows a dosing regimen of 2 times/day.
Distribution
There is no significant cumulation when the drug is taken repeatedly.
Metabolism
Meberine hydrochloride is primarily metabolized by esterases, which first break down the ester into veric acid and meberine alcohol. The main metabolite circulating in plasma is demethylated carboxylic acid. The T1/2 in equilibrium of demethylated carboxylic acid is approximately 5.77 h. When repeatedly taken at a dose of 200 mg 2 times/day, the Cmax of demethylated carboxylic acid in the blood is 804 ng/ml, Tmax is about 3 h.
The average relative bioavailability of the drug in a modified-release capsule is 97%.
Elimination
Meberine is not excreted unchanged, it is completely metabolized; its metabolites are almost completely eliminated from the body. Veratroic acid is excreted by the kidneys. Alcohol of meberine is also excreted by the kidneys, partly as carboxylic acid and partly as demethylated carboxylic acid.
Indications
Indications
Duspatalin® is used in adults over 18 years of age for
• symptomatic treatment of pain, spasms, dysfunction and discomfort in the intestinal area associated with irritable bowel syndrome;
• symptomatic treatment of spasms of the gastrointestinal tract (including those caused by organic diseases).
Special instructions
Special instructions
Not applicable.
Children and teenagers
Do not give this medicine to children and adolescents under 18 years of age. The safety and effectiveness of Duspatalin® in children under 18 years of age have not yet been established.
Driving vehicles and working with machinery
Experience with the drug does not indicate any adverse effect of mebeverine on the ability to drive a car or use other mechanisms.
Active ingredient
Active ingredient
Mebeverine
Composition
Composition
The active ingredient is mebeverine.
Each capsule contains 200 mg mebeverine (as hydrochloride).
Other ingredients (excipients) are magnesium stearate, methyl methacrylate and ethyl acrylate copolymer [1:2] 30% dispersion (30% polyacrylate dispersion), talc, hypromellose, methacrylic acid and ethyl acrylate copolymer [1:1] 30% dispersion, triacetin, gelatin, titanium dioxide (E 171), ink (shellac (E 904), propylene glycol, aqueous ammonia, potassium hydroxide, black iron oxide dye (E 172)).
Pregnancy
Pregnancy
If you are pregnant or breastfeeding, think you may be pregnant, or are planning a pregnancy, consult your doctor before starting to use the drug.
Pregnancy
Experience with the use of mebeverine in pregnant women is limited. Do not take Duspatalin® during pregnancy without consulting your doctor.
Breastfeeding
Do not take Duspatalin® if you are breastfeeding or plan to breastfeed. It is not known whether mebeverine or its metabolites passes into breast milk. If you need to take the drug during breastfeeding, you should temporarily stop feeding your baby breast milk.
Contraindications
Contraindications
Do not take Duspatalin®:
• if you are allergic to mebeverine or any of the other ingredients of this medicine (listed in section 6 of the leaflet);
• if you are under 18 years of age;
• if you are pregnant.
Side Effects
Side Effects
Like all medicines, Duspatalin® can cause side effects, although not everyone gets them.
Stop taking Duspatalin® and seek immediate medical attention if you experience any of the following severe adverse reactions:
• difficulty breathing, rapid pulse, sharp decrease in blood pressure (weakness and dizziness), sweating (anaphylactic reactions);
• difficulty breathing, swelling of the face, neck, lips, tongue, throat (angioedema, including the face).
Other possible adverse reactions that may occur while taking Duspatalin®
• urticaria (allergic rash),
• exanthema (skin rash),
• allergic reactions.
Interaction
Interaction
Tell your doctor if you are taking, have recently taken, or may start taking any other medications.
Overdose
Overdose
If you accidentally take too many Duspatalin® capsules, consult your doctor immediately. If possible, show your doctor the package of Duspatalin®.
Storage conditions
Storage conditions
Store at a temperature not exceeding 25 °C.
Manufacturer
Manufacturer
Veropharm LLC, Russia
Additional information
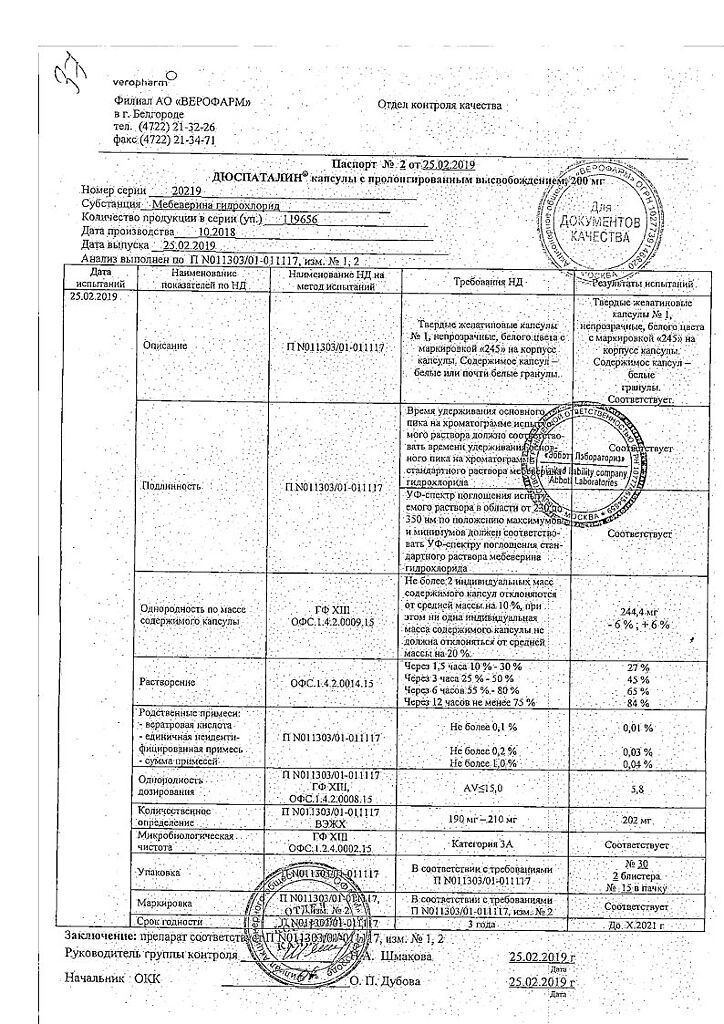
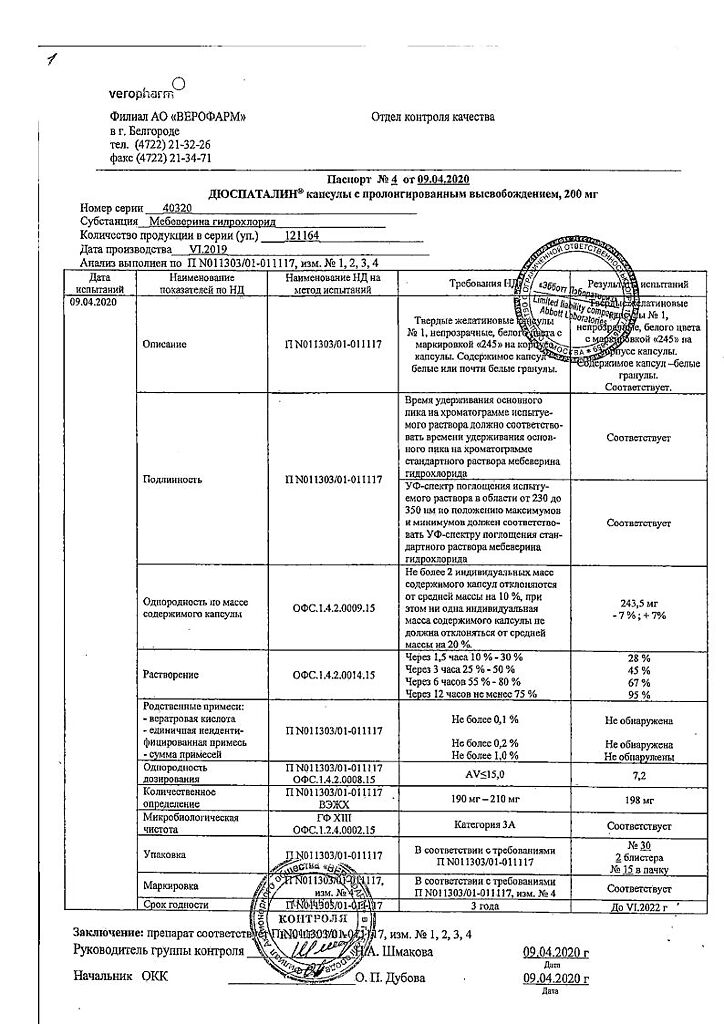
| Shelf life | 3 years. Do not use after the expiration date. |
|---|---|
| Conditions of storage | List B. Store at a temperature not exceeding 25°C. Keep out of reach of children! |
| Manufacturer | Veropharm AO, Russia |
| Medication form | slow-release capsules |
| Brand | Veropharm AO |
Other forms…
Related products
Buy Duspatalin, capsules 200 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.