No products in the cart.
Detralex, 1000 mg 18 pcs.
€27.54 €22.95
Description
Pharmacodynamics
Detralex® has venotonic and angioprotective properties. The drug reduces venous distensibility and venous stasis, reduces capillary permeability and increases their resistance. The results of clinical studies confirm the pharmacological activity of the drug with respect to the indices of venous hemodynamics. Statistically significant dose-dependent effect of the drug Detralex® has been shown for the following venous plethysmographic parameters:
- venous capacity,
- venous distensibility,
- time of venous emptying.
The optimal ″dose-effect ratio″ is observed when taking 1000 mg daily. Detralex® increases venous tone: venous occlusion plethysmography has shown a decrease in venous emptying time. In patients with signs of severe microcirculatory disorders, after therapy with Detralex® an increase in capillary resistance evaluated by angiostereometry (statistically significant compared to placebo) has been observed. The therapeutic efficacy of Detralex® has been proven in treatment of chronic diseases of lower limb veins and hemorrhoids.
Pharmacokinetics
The main excretion of the drug is in the feces. With the urine, on average, about 14% of the taken amount of the drug is excreted. The elimination half-life is 11 hours. The drug undergoes active metabolism, which is confirmed by the presence of phenolic acids in the urine.
Indications
Indications
Detralex® is indicated for the treatment of symptoms of chronic venous diseases (elimination and relief of symptoms).
Treatment of symptoms of venous-lymphatic insufficiency:
pain;
spasms of the lower extremities;
feeling of heaviness and fullness in the legs;
“tired” legs.
Treatment of manifestations of venous-lymphatic insufficiency:
swelling of the lower extremities;
trophic changes in the skin and subcutaneous tissue;
venous trophic ulcers.
Symptomatic treatment of acute and chronic hemorrhoids.
Pharmacological effect
Pharmacological effect
Pharmacodynamics
Detralex® has venotonic and angioprotective properties. The drug reduces the distensibility of veins and venous stagnation, reduces capillary permeability and increases their resistance. The results of clinical studies confirm the pharmacological activity of the drug in relation to indicators of venous hemodynamics. A statistically significant dose-dependent effect of the drug Detralex® was demonstrated for the following venous plethysmographic parameters:
venous capacity,
venous distensibility,
time of venous emptying.
The optimal dose-effect ratio is observed when taking 1000 mg per day. Detralex® increases venous tone: using venous occlusion plethysmography, a decrease in the time of venous emptying was shown. In patients with signs of severe microcirculation disorders, after treatment with Detralex® there is a (statistically significant compared to placebo) increase in capillary resistance, assessed by angiostereometry. The therapeutic effectiveness of the drug Detralex® has been proven in the treatment of chronic diseases of the veins of the lower extremities, as well as in the treatment of hemorrhoids.
Pharmacokinetics
The main excretion of the drug occurs in the feces. On average, about 14% of the administered amount of the drug is excreted in the urine. The half-life is 11 hours. The drug undergoes active metabolism, which is confirmed by the presence of phenolic acids in the urine.
Special instructions
Special instructions
Before you start taking Detralex®, it is recommended to consult your doctor.
In the treatment of hemorrhoids, the use of Detralex® does not replace the specific treatment of other diseases of the rectum and anal canal. When using the drug on your own, do not exceed the maximum periods and recommended doses specified in the “Method of administration and dosage” section. If symptoms of hemorrhoids persist after the recommended course of therapy, you should be examined by a proctologist, who will select further therapy.
In the presence of venous circulation disorders, the maximum effect of treatment is ensured by a combination of therapy with additional therapeutic and preventive measures / a healthy (balanced) lifestyle: it is advisable to avoid long exposure to the sun, prolonged standing, and it is also recommended to reduce excess body weight. Walking and, in some cases, wearing special stockings helps improve blood circulation.
Consult your doctor immediately if, during treatment, your condition worsens or your symptoms do not improve.
Impact on the ability to drive a car and perform work that requires high speed of mental and physical reactions:
Clinical studies have not been conducted to study the effect of the drug Detralex® on the ability to drive a car and perform work that requires a high speed of mental and physical reactions. However, based on the available safety data, we can conclude that Detralex® does not affect (has no significant effect) on these processes.
Active ingredient
Active ingredient
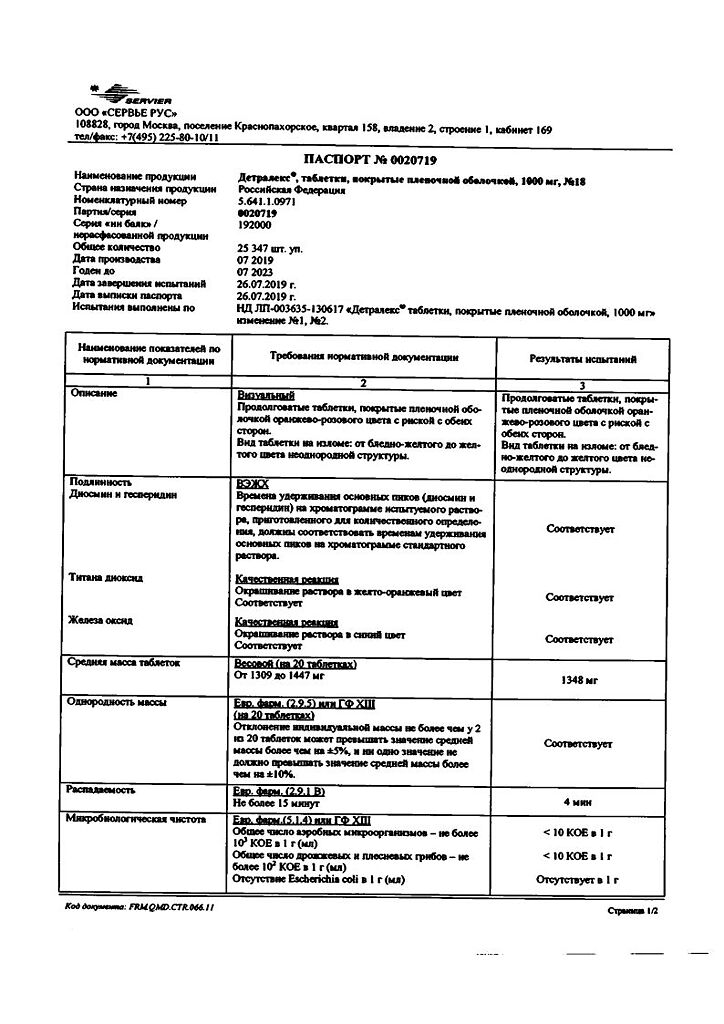
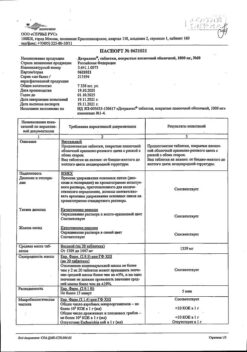
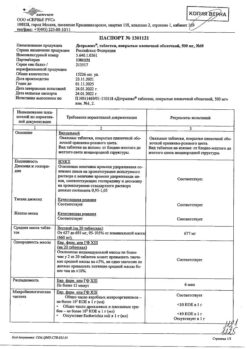
Purified micronized flavonoid fraction (diosmin, flavonoids in terms of hesperidin)
Composition
Composition
One film-coated tablet contains:
Active ingredient:
1000 mg of purified micronized flavonoid fraction, consisting of diosmin 900 mg (90%) and flavonoids, calculated as hesperidin 100 mg (10%).
Excipients:
purified water, gelatin, magnesium stearate, microcrystalline cellulose, sodium carboxymethyl starch type A, talc.
Film shell:
sodium lauryl sulfate, macrogol 6000, orange-pink premix for film shell, consisting of: glycerol, magnesium stearate, hypromellose, macrogol 6000, magnesium stearate, red iron oxide dye, titanium dioxide, yellow iron oxide dye.
Auxiliary substance for polishing tablets:
Macrogol 6000 1,300 mg. The mass of the film-coated tablet is 1378.425 mg. 1) Taking into account the average moisture content of the purified, micronized flavonoid fraction – 4%, (or 40 mg per tablet), the amount of substance per tablet is 1040.00 mg.
Pregnancy
Pregnancy
Pregnancy:
There are no or limited data on the use of purified micronized flavonoid fraction in pregnant women.
Animal studies have not shown reproductive toxicity.
As a precautionary measure, it is preferable not to use Detralex® during pregnancy.
Breastfeeding period:
It is unknown whether the purified micronized flavonoid fraction (metabolites) passes into human breast milk.
A risk for newborns and infants cannot be excluded. It is necessary to make a decision either to stop breastfeeding or to cancel therapy with Detralex®, taking into account the benefits of breastfeeding for the child and the benefits of therapy for the woman.
Effect on reproductive function:
Reproductive toxicity studies showed no effect on reproductive function in rats of either sex.
Contraindications
Contraindications
Hypersensitivity to the active components or excipients included in the drug.
Pregnancy and breastfeeding (limited or no experience with use).
Children under 18 years of age (no experience of use).
Side Effects
Side Effects
Side effects of Detralex® observed during clinical trials were mild. Disorders from the gastrointestinal tract (diarrhea, dyspepsia, nausea, vomiting) were predominantly observed. While taking the drug Detralex®, the following side effects were reported, in the following gradation: very often (≥1/10); often (≥1/100, <1/10); uncommon (≥1/1,000, <1/100); rare (≥1/10,000, <1/1,000); extremely rare (<1/10,000), unspecified frequency (frequency cannot be calculated from available data).
From the central nervous system:
Rarely: dizziness, headache, general malaise.
From the gastrointestinal tract:
Common: diarrhea, dyspepsia, nausea, vomiting.
Uncommon: colitis. Unspecified frequency: abdominal pain.
From the skin and subcutaneous tissues: Rarely: skin rash, itching, urticaria. Unspecified frequency: isolated swelling of the face, lips, eyelids. In exceptional cases, angioedema.
INFORM YOUR DOCTOR ABOUT ANY ADVERSE REACTIONS AND SENSATIONS YOU EXPERIENCE, INCLUDING THOSE NOT MENTIONED IN THIS INSTRUCTION, AND ALSO ABOUT CHANGES IN LABORATORY VALUES DURING THE THERAPY.
Interaction
Interaction
Clinical studies have not been conducted to study the interaction of the drug Detralex® with other drugs. To date, no cases of drug interactions have been reported.
YOU SHOULD INFORM YOUR DOCTOR ABOUT ALL MEDICINES YOU ARE TAKEN.
Overdose
Overdose
Symptoms
Data on cases of overdose of Detralex® are limited. The most common adverse reactions in cases of overdose were gastrointestinal disorders (diarrhea, nausea, abdominal pain) and skin reactions (itching, rash).
Treatment
Help in case of overdose should consist of eliminating clinical symptoms.
In case of overdose of the drug, you should immediately consult a doctor.
Storage conditions
Storage conditions
At a temperature not higher than 30°C.
Keep out of the reach of children.
Shelf life
Shelf life
4 years.
Do not use after the expiration date stated on the package.
Manufacturer
Manufacturer
Servier Rus LLC, Russia
Additional information
| Shelf life | 4 years. Do not use after the expiration date stated on the package. |
|---|---|
| Conditions of storage | At a temperature not exceeding 30°C. Keep out of reach of children. |
| Manufacturer | Servier Rus LLC, Russia |
| Medication form | pills |
| Brand | Servier Rus LLC |
Other forms…
Related products
Buy Detralex, 1000 mg 18 pcs. with delivery to USA, UK, Europe and over 120 other countries.