No products in the cart.
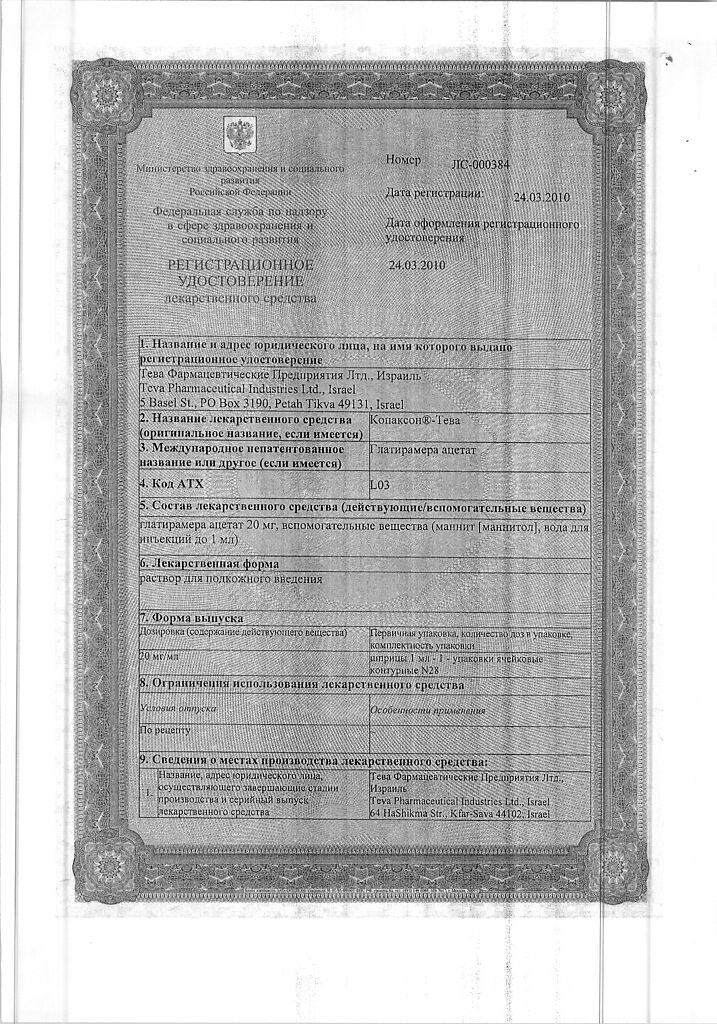
Copaxone-Teva, 20 mg/ml 1 ml syringes 28 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
Copaxone-Teva (glatiramer acetate) is the acetic acid salt of a mixture of synthetic polypeptides formed by 4 natural amino acids: L-glutamic acid, L-alanine, L-tyrosine and L-lysine, and by chemical structure has elements similar to the main myelin protein.
Glatiramer acetate reverses the course of the pathological process in the demyelinating CNS disease multiple sclerosis, which is an autoimmune disease that changes the ratio of T-suppressors in the body. Glatiramer acetate has an immunomodulatory effect at the injection site. Its therapeutic effect is mediated through systemic distribution of activated T-suppressors.
Glatiramer acetate has a specific mechanism of action based on the ability to competitively displace myelin antigens – myelin basic protein, myelin oligodendrocytic glycoprotein and proteolipid protein in the binding sites with molecules of the main histocompatibility complex class 2, located on antigen-presenting cells. The consequence of competitive displacement is two responses: stimulation of antigen-specific suppressor T lymphocytes (Th2-type) and inhibition of antigen-specific effector T lymphocytes (Th1-type). Activated T-suppressor lymphocytes enter the systemic circulation and penetrate into the CNS.
Once at the site of inflammation in the CNS, these T-lymphocytes are reactivated by myelin antigens, resulting in their production of anti-inflammatory cytokines (including IL-4, IL-6, IL-10). These cytokines reduce local inflammation by suppressing the local inflammatory T-cell response, leading to the accumulation of specific anti-inflammatory Th2-type cells and inhibition of the pro-inflammatory Th1-cell system.
In addition, glatiramer acetate stimulates synthesis of neurotrophic factor by Th2 cells and protects brain structures from damage (neuroprotective effect). Glatiramer acetate has no generalized effect on the main parts of the normal immune reactions of the body, which distinguishes it in principle from nonspecific immunomodulators, including beta-interferon drugs. Formed antibodies to glatiramer acetate do not have a neutralizing effect during long-term use, which reduces the clinical effect of the drug.
Indications
Indications
clinically isolated syndrome (the only clinical episode of demyelination, suggesting multiple sclerosis) with severity of the inflammatory process, requiring the use of intravenous corticosteroids (to slow down the transition to clinically significant multiple sclerosis);
relapsing-remitting multiple sclerosis (to reduce the frequency of exacerbations, slow down the development of disabling complications).
Pharmacological effect
Pharmacological effect
Copaxone-Teva (glatiramer acetate) is the acetic acid salt of a mixture of synthetic polypeptides formed by 4 natural amino acids: L-glutamic acid, L-alanine, L-tyrosine and L-lysine, and its chemical structure has elements of similarity to myelin basic protein.
Glatiramer acetate changes the course of the pathological process in the demyelinating disease of the central nervous system – multiple sclerosis, which is an autoimmune disease that changes the ratio of T-suppressors in the body. Glatiramer acetate has an immunomodulatory effect at the injection site. Its therapeutic effect is mediated through the systemic distribution of activated T-suppressor cells.
Glatiramer acetate has a specific mechanism of action, which is based on the ability to competitively replace myelin antigens – myelin basic protein, myelin oligodendrocyte glycoprotein and proteolipid protein at the sites of binding to molecules of the major histocompatibility complex class 2 located on antigen-presenting cells. The consequence of competitive displacement is two reactions: stimulation of antigen-specific suppressor T-lymphocytes (Th2-type) and inhibition of antigen-specific effector T-lymphocytes (Th1-type). Activated T-suppressor lymphocytes enter the systemic circulation and penetrate the central nervous system.
Once in the site of inflammation in the central nervous system, these T-lymphocytes are reactivated by myelin antigens, which leads to their production of anti-inflammatory cytokines (including IL-4, IL-6, IL-10). These cytokines reduce local inflammation by suppressing the local inflammatory T-cell response, leading to the accumulation of specific anti-inflammatory Th2-type cells and inhibition of the pro-inflammatory Th1 cell system.
In addition, glatiramer acetate stimulates the synthesis of neurotrophic factor by Th2 cells and protects brain structures from damage (neuroprotective effect). Glatiramer acetate does not have a generalized effect on the main components of the body’s normal immune reactions, which fundamentally distinguishes it from nonspecific immunomodulators, including beta-interferon drugs. The antibodies formed to glatiramer acetate during long-term use do not have a neutralizing effect, which reduces the clinical effect of the drug.
Special instructions
Special instructions
The drug should be prescribed with caution to patients predisposed to allergic reactions and with heart disease.
Patients with impaired renal function should regularly monitor laboratory parameters.
The patient should be informed about the self-injection technique for the safe use of Copaxone-Teva and receive instructions on the use of antiseptic methods when preparing the injection solution and its administration. The first injection should be carried out under the supervision of a qualified specialist.
It is necessary to periodically monitor the patient’s understanding of the importance of using antiseptic treatment during self-injections. Patients should be informed about the inadmissibility of reusing needles and syringes, as well as the procedure for their safe disposal. Used needles and syringes should be placed in hard packaging and only then thrown away. Patients should be informed about possible adverse reactions associated with the use of the drug.
Impact on the ability to drive vehicles and operate machinery
Based on available data, there is no need for special precautions for persons operating motor vehicles or complex machinery.
Copaxone-Teva is compatible with corticosteroid hormones.
Active ingredient
Active ingredient
Glatiramer acetate
Composition
Composition
1 ml (1 syringe) contains:
Contraindications
Contraindications
hypersensitivity to glatiramer acetate or mannitol;
pregnancy;
children under 18 years of age (efficacy and safety have not been studied).
With caution: predisposition to the development of allergic reactions; cardiovascular diseases; renal dysfunction.
Side Effects
Side Effects
Immediately after the injection, the following are possible:
Local reactions: pain, redness, swelling; rarely – atrophy of the skin or subcutaneous tissue at the injection site, abscess, hematoma.
Systemic reactions: flushing, chest pain, rapid heartbeat, anxiety, shortness of breath, difficulty swallowing, urticaria. These symptoms may be temporary and limited in nature and do not require special intervention; they may begin several months after the start of therapy, and the patient may experience one or another symptom episodically.
Sometimes you may experience:
From the cardiovascular system: palpitations, vasodilation; rarely – syncope, increased blood pressure, extrasystole, pallor, varicose veins.
From the digestive system: constipation, diarrhea, nausea; very rarely – anorexia, dysphagia, gastroenteritis, stomatitis, caries.
Allergic reactions: anaphylactic reactions, including shock.
From the blood and lymphatic system: rarely – lymphadenopathy; very rarely – eosinophilia, splenomegaly.
Metabolism: very rarely – edema, weight loss, aversion to alcohol.
From the musculoskeletal system: rarely – arthralgia, arthritis.
From the nervous system: rarely – emotional lability, clouding of consciousness (stupor), convulsions, anxiety, depression, dizziness, tremor, ataxia, headache.
From the respiratory system: rarely – increased breathing (hyperventilation); in isolated cases – bronchospasm, nosebleeds, hypoventilation, voice change.
From the reproductive system: rarely – amenorrhea, impotence, menorrhagia, vaginal bleeding.
Other: rarely – hematuria.
Interaction
Interaction
The interaction between Copaxone-Teva and other drugs has not been sufficiently studied.
During clinical trials, no significant drug interactions were identified, including the simultaneous use of Copaxone-Teva with drugs used to treat multiple sclerosis, incl. with corticosteroids (when used simultaneously for up to 28 days).
It is extremely rare that the frequency of local reactions may increase.
Overdose
Overdose
There is no data on an overdose of Copaxone-Teva.
Storage conditions
Storage conditions
At 2–8 °C
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Teva Pharmaceutical Enterprises Ltd., Israel
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | At 2-8 °C |
| Manufacturer | Teva Pharmaceutical Enterprises Ltd, Israel |
| Medication form | solution |
| Brand | Teva Pharmaceutical Enterprises Ltd |
Related products
Buy Copaxone-Teva, 20 mg/ml 1 ml syringes 28 pcs with delivery to USA, UK, Europe and over 120 other countries.