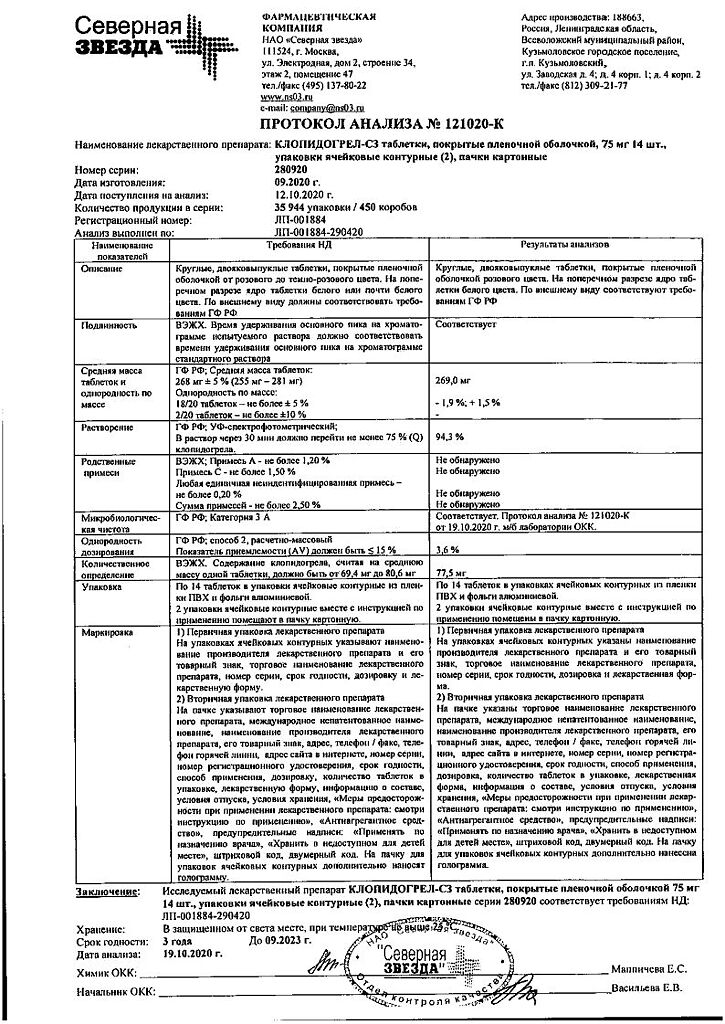
No products in the cart.
Clopidogrel-SZ, 75 mg 28 pcs
€12.37 €10.31
Out of stock
(E-mail when Stock is available)
Description
Pharmacotherapeutic group:
anti-aggregant.
The ATX code: [B01AC04]
Pharmacological Properties
Pharmacodynamics
Clopidogrel is a prodrug, one of whose active metabolites is a platelet aggregation inhibitor. Active metabolite of clopidogrel selectively inhibits binding of adenosine diphosphate (ADP) to P2Y12 receptor of platelets and subsequent ADP-mediated activation of GPIIb/IIIa complex, leading to suppression of platelet aggregation. Due to irreversible binding, platelets remain immune to ADP stimulation for the remainder of their lives (approximately 7-10 days), and recovery of normal platelet function occurs at a rate consistent with the rate of platelet renewal. Platelet aggregation caused by agonists other than ADP is also inhibited by blocking enhanced platelet activation by the released ADP. Since the formation of the active metabolite occurs via P450 system isoenzymes, some of which may be polymorphic or may be inhibited by other drugs, adequate platelet suppression is not possible in all patients. Clopidogrel is able to prevent the development of atherothrombosis in any localization of atherosclerotic vascular lesions, in particular in lesions of the cerebral, coronary or peripheral arteries.
When clopidogrel is taken daily at a dose of 75 mg a significant suppression of ADP-induced platelet aggregation is noted from the first day of administration, which gradually increases over 3-7 days and then reaches a constant level (when equilibrium state is reached). In the equilibrium state, platelet aggregation is suppressed by an average of 40-60%. After stopping clopidogrel administration, platelet aggregation and bleeding time gradually return to the initial level on average within 5 days.
Pharmacokinetics
Eabsorption
In a course of oral administration at a dose of 75 mg daily, clopidogrel is rapidly absorbed. Mean maximum plasma concentrations of unchanged clopidogrel (approximately 2.2-2.5 ng/ml after oral administration of a single dose of 75 mg) are reached approximately 45 minutes after the dose. According to the excretion of clopidogrel metabolites by the kidneys, its absorption is approximately 50%.
Distribution
In vitro clopidogrel and its major circulating inactive metabolite are reversibly bound to plasma proteins (98% and 94%, respectively) and this binding is unsaturated up to a concentration of 100 mg/L.
Metabolism
Clopidogrel is extensively metabolized in the liver. In vitro and in vivo clopidogrel is metabolized in two ways: the first – through enzymes and subsequent hydrolysis to form inactive carboxylic acid derivative (85 % of circulating metabolites), and the second way – through cytochrome P450 system. First, clopidogrel is metabolized to 2-oxo-clopidogrel, which is an intermediate metabolite. Subsequent metabolism of 2-oxo-clopidogrel leads to the formation of the active metabolite of clopidogrel – thiol derivative of clopidogrel. In vitro, this metabolic pathway occurs via the isoenzymes CYP2CI9, CYP1A2, and CYP2B6. The active thiol metabolite of clopidogrel, which has been isolated in in vitro studies, binds rapidly and irreversibly to platelet receptors, thus blocking platelet aggregation.
Elimination
For 120 hours after oral human ingestion of 14C-labeled clopidogrel, approximately 50% of the radioactivity is excreted by the kidneys and approximately 46% of the radioactivity is excreted through the intestine. After a single oral dose of 75 mg, the half-life of clopidogrel is about 6 hours. After a single dose and repeated doses, the half-life of the main circulating inactive metabolite in blood is 8 hours.
Pharmacogenetics
Both the active metabolite and the intermediate metabolite 2-oxo-clopidogrel are formed using the CYP2C19 isoenzyme. The pharmacokinetics and antiaggregant effect of the active metabolite clopidogrel, in an ex vivo study of platelet aggregation, vary depending on the genotype of the CYP2C19 isoenzyme. The CYP2C19*1 gene allele corresponds to fully functional metabolism, whereas the CYP2C19*2 and CYP2C19*3 gene alleles are nonfunctional. The CYP2C19*2 and CYP2C19*3 gene alleles are responsible for reduced metabolism in the majority of Caucasoid (85%) and Mongoloid races (99%). Other alleles that are associated with absence or reduced metabolism are less common and include, but are not limited to, CYP2C19*4, *5, *6, *7, and *8 alleles. Patients with low CYP2C19 isoenzyme activity, must have the two loss-of-function gene alleles listed above. Published frequencies of phenotypes of individuals with low CYP2C19 isoenzyme activity are 2% in Caucasians, 4% in non-Hispanics, and 14% in Chinese.
Tests are available to determine if a patient has the CYP2C19 isoenzyme genotype. According to a cross-sectional study (40 volunteers) and a meta-analysis of six studies (335 volunteers). which included subjects with very high, high, intermediate, and low CYP2C19 isoenzyme activity, no significant differences in exposure to the active metabolite and in the mean values of platelet aggregation inhibition (IAT) (induced by ADP) were found in volunteers with very high, high, and intermediate CYP2C19 isoenzyme activity. Volunteers with low CYP2C19 isoenzyme activity had decreased exposure to the active metabolite compared to volunteers with high CYP2C19 isoenzyme activity.
When volunteers with low CYP2C19 isoenzyme activity received the 600 mg loading dose/150 mg maintenance dose (600 mg/150 mg) treatment regimen, exposure to the active metabolite was higher than when receiving the 300 mg/75 mg treatment regimen. In addition, IAT was similar to that in the groups of patients with higher metabolism rates by the CYP2C19 isoenzyme receiving the 300 mg/75 mg treatment regimen. However, the dosing regimen of clopidogrel for patients in this group (patients with low CYP2C19 isoenzyme activity) has not yet been established in studies considering clinical outcomes. Clinical studies conducted to date have not had sufficient sample size to detect differences in clinical outcome in patients with low CYP2C19 isoenzyme activity.
Particular patient groups
The pharmacokinetics of the active metabolite of clopidogrel in elderly patients, children, patients with renal and hepatic diseases have not been studied.
Indications
Indications
– Prevention of atherothrombotic events in patients who have suffered a myocardial infarction (with a duration of several days to 35 days), ischemic stroke (with a duration of 7 days to 6 months) or have been diagnosed with occlusive peripheral artery disease.
– Prevention of atherothrombotic events (in combination with acetylsalicylic acid) in patients with acute coronary syndrome:
– without ST segment elevation (unstable angina or non-Q wave myocardial infarction), including patients who underwent stenting during percutaneous coronary intervention;
– with ST segment elevation (acute myocardial infarction) with drug treatment and the possibility of thrombolysis.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group:
antiplatelet agent.
ATX code: [B01AC04]
PHARMACOLOGICAL PROPERTIES
Pharmacodynamics
Clopidogrel is a prodrug, one of the active metabolites of which is an inhibitor of platelet aggregation. The active metabolite of clopidogrel selectively inhibits the binding of adenosine diphosphate (ADP) to the P2Y12 platelet receptor and subsequent ADP-mediated activation of the GPIIb/IIIa complex, leading to suppression of platelet aggregation.
Due to irreversible binding, platelets remain immune to ADP stimulation for the remainder of their life (approximately 7-10 days), and restoration of normal platelet function occurs at a rate consistent with platelet turnover.
Platelet aggregation induced by agonists other than ADP is also inhibited by blocking enhanced platelet activation by released ADP. Since the formation of the active metabolite occurs with the help of isoenzymes of the P450 system, some of which may differ in polymorphism or may be inhibited by other drugs, adequate platelet suppression is not possible in all patients.
Clopidogrel is able to prevent the development of atherothrombosis in any localization of atherosclerotic vascular lesions, in particular in lesions of the cerebral, coronary or peripheral arteries.
When taking clopidogrel daily at a dose of 75 mg, from the first day of administration there is a significant suppression of ADP-induced platelet aggregation, which gradually increases over 3-7 days and then reaches a constant level (when an equilibrium state is reached). At steady state, platelet aggregation is suppressed by an average of 40-60%. After discontinuation of clopidogrel, platelet aggregation and bleeding time gradually returned to baseline levels within an average of 5 days.
Pharmacokinetics
Suction
When administered orally at a dose of 75 mg per day, clopidogrel is rapidly absorbed. Average maximum plasma concentrations of unchanged clopidogrel (approximately 2.2-2.5 ng/ml after oral administration of a single 75 mg dose) are achieved approximately 45 minutes after administration. According to the excretion of clopidogrel metabolites by the kidneys, its absorption is approximately 50%.
Distribution
In vitro, clopidogrel and its main inactive metabolite circulating in the blood are reversibly bound to plasma proteins (98% and 94%, respectively) and this binding is unsaturated up to a concentration of 100 mg/l.
Metabolism
Clopidogrel is extensively metabolized in the liver. In vitro and in vivo, clopidogrel is metabolized in two ways: the first – through enzymes and subsequent hydrolysis with the formation of an inactive carboxylic acid derivative (85% of circulating metabolites), and the second way – through the cytochrome P450 system. Initially, clopidogrel is metabolized to 2-oxo-clopidogrel, which is an intermediate metabolite.
Subsequent metabolism of 2-oxo-clopidogrel leads to the formation of the active metabolite of clopidogrel – the thiol derivative of clopidogrel. In vitro, this metabolic pathway occurs through the isoenzymes CYP2CI9, CYP1A2 and CYP2B6. The active thiol metabolite of clopidogrel, which was isolated in in vitro studies, rapidly and irreversibly binds to platelet receptors, thereby blocking platelet aggregation.
Removal
Within 120 hours after human ingestion of 14C-labeled clopidogrel, approximately 50% of the radioactivity is excreted by the kidneys and approximately 46% of the radioactivity is excreted through the intestines. After a single oral dose of 75 mg, the half-life of clopidogrel is approximately 6 hours. After a single dose and repeated doses, the half-life of the main inactive metabolite circulating in the blood is 8 hours.
Pharmacogenetics
With the help of the CYP2C19 isoenzyme, both the active metabolite and the intermediate metabolite, 2-oxo-clopidogrel, are formed. The pharmacokinetics and antiplatelet effect of the active metabolite of clopidogrel, when studying platelet aggregation ex vivo, vary depending on the genotype of the CYP2C19 isoenzyme.
The allele of the CYP2C19*1 gene corresponds to a fully functional metabolism, while the alleles of the CYP2C19*2 and CYP2C19*3 genes are non-functional. Alleles of the CYP2C19*2 and CYP2C19*3 genes are the cause of decreased metabolism in the majority of representatives of the Caucasian (85%) and Mongoloid races (99%). Other alleles associated with absent or decreased metabolism are less common and include, but are not limited to, the CYP2C19*4, *5, *6, *7, and *8 alleles. Patients with low CYP2C19 activity must have the two loss-of-function alleles listed above.
Published frequencies of occurrence of phenotypes in individuals with low CYP2C19 activity are 2% in Caucasians, 4% in Blacks, and 14% in Chinese.
To determine the patient’s genotype of the CYP2C19 isoenzyme, there are appropriate tests. Based on a cross-sectional study (40 volunteers) and a meta-analysis of six studies (335 volunteers). In the study, which included individuals with very high, high, intermediate and low CYP2C19 activity, there were no significant differences in the exposure of the active metabolite and in the mean values of inhibition of platelet aggregation (PAI) (induced by ADP) in volunteers with very high, high and intermediate CYP2C19 activity.
In volunteers with low CYP2C19 isoenzyme activity, exposure to the active metabolite was reduced compared to volunteers with high CYP2C19 isoenzyme activity.
When volunteers with low CYP2C19 activity received the 600 mg loading dose/150 mg maintenance dose regimen (600 mg/150 mg), exposure to the active metabolite was higher than when taking the 300 mg/75 mg regimen.
In addition, IAT was similar to that in groups of patients with higher rates of metabolism by the isoenzyme CYP2C19 receiving the 300 mg/75 mg treatment regimen. However, in studies taking into account clinical outcomes, the dosage regimen of clopidogrel for patients in this group (patients with low CYP2C19 isoenzyme activity) has not yet been established.
Clinical studies conducted to date have not had a sufficient sample size to detect differences in clinical outcome in patients with low CYP2C19 activity.
Selected patient groups
The pharmacokinetics of the active metabolite of clopidogrel in elderly patients, children, and patients with kidney and liver diseases has not been studied.
Special instructions
Special instructions
When treating with Clopidogrel-SZ, especially during the first weeks of therapy and/or after invasive cardiac procedures/surgery, it is necessary to carefully monitor patients to exclude signs of bleeding, incl. and hidden.
Due to the risk of bleeding and hematological undesirable effects, if clinical symptoms suspicious for bleeding appear during treatment, you should urgently do a clinical blood test, determine APTT, platelet count, indicators of platelet functional activity and conduct other necessary studies.
Clopidogrel-SZ, as well as other antiplatelet drugs, should be used with caution in patients who have an increased risk of bleeding associated with trauma, surgery or other pathological conditions, as well as in patients receiving acetylsalicylic acid, NSAIDs, incl. COX-2 inhibitors, heparin or glycoprotein IIb/IIIa inhibitors.
The combined use of clopidogrel with warfarin may increase the intensity of bleeding, therefore, with the exception of very rare clinical situations (such as the presence of a floating thrombus in the left ventricle, stenting in patients with atrial fibrillation), the combined use of clopidogrel and warfarin is not recommended.
If the patient is undergoing a planned surgical intervention, and there is no need for an antiplatelet effect, then 7 days before surgery, the drug Clopidogrel-SZ should be stopped.
Clopidogrel prolongs bleeding time and should be used with caution in patients with diseases predisposing to the development of bleeding (especially gastrointestinal and intraocular).
Patients should be warned that when using Clopidogrel-SZ (alone or in combination with acetylsalicylic acid) it may take longer to stop bleeding, and that if they experience unusual bleeding (in location or duration) they should inform their doctor. Before any upcoming surgery and before starting any new drug, patients should tell their doctor (including dentist) that they are taking Clopidogrel-SZ.
Very rarely, after using the drug Clopidogrel-SZ (sometimes even for a short time), there have been cases of development of thrombocytopenic thrombohemolytic purpura (THP), which is characterized by thrombocytopenia and microangiopathic hemolytic anemia, accompanied by neurological disorders, renal dysfunction and fever. THP is a potentially life-threatening condition that requires immediate treatment, including plasmapheresis.
During treatment, it is necessary to monitor the functional activity of the liver. In case of severe liver dysfunction, one should remember the risk of developing hemorrhagic diathesis.
Taking the drug Clopidogrel-SZ is not recommended for acute stroke less than 7 days old (since there is no data on its use in this condition).
Clopidogrel-SZ should not be taken by patients with rare hereditary galactose intolerance, lactase deficiency and glucose-galactose malabsorption syndrome.
Impact on the ability to drive vehicles and operate machinery
Clopidogrel-SZ does not have a significant effect on the ability required to drive vehicles or operate machinery.
Active ingredient
Active ingredient
Clopidogrel
Composition
Composition
1 film-coated tablet contains:
active ingredient:
clopidogrel hydrosulfate equivalent to clopidogrel or clopidogrel bisulfate equivalent to clopidogrel – 75 mg
excipients (core):
lactose monohydrate (milk sugar) – 38.4 mg, microcrystalline cellulose – 129.48 mg, croscarmellose sodium (primellose) – 12.0 mg, colloidal silicon dioxide (aerosil) – 3.12 mg, sodium stearyl fumarate – 2.0 mg
excipients (shell):
Opadry II – 8 mg (polyvinyl alcohol, partially hydrolyzed – 3.52 mg, talc – 1.6 mg, titanium dioxide E 171 – 1.5336 mg, macrogol (polyethylene glycol 3350) – 0.988 mg, soy lecithin E 322 – 0.28 mg, aluminum varnish based on azorubine dye – 0.0408 mg, aluminum varnish based on crimson dye [Ponceau 4R] – 0.0328 mg, aluminum varnish based on indigo carmine dye – 0.0048 mg).
Pregnancy
Pregnancy
Due to the lack of data, it is not recommended to take clopidogrel during pregnancy and lactation.
Contraindications
Contraindications
– Hypersensitivity to clopidogrel or any of the excipients of the drug. -Severe liver failure.
– Acute bleeding, such as bleeding from a peptic ulcer or intracranial hemorrhage.
– Rare hereditary lactose intolerance, lactase deficiency and glucose-galactose malabsorption.
– Pregnancy and lactation (see “Pregnancy and breastfeeding”).
– Children under 18 years of age (safety and effectiveness of use have not been established).
With caution
– Moderate liver failure, which may predispose to bleeding (limited clinical experience with use).
– Renal failure (limited clinical experience).
– Trauma, surgical interventions (see “Special instructions”).
– Diseases in which there is a predisposition to the development of bleeding (especially gastrointestinal or intraocular).
– Simultaneous use of non-steroidal anti-inflammatory drugs, including selective inhibitors of cyclooxygenase-2 (COX-2).
– Simultaneous administration of warfarin, heparin, glycoprotein IIb/IIIa inhibitors.
– In patients with a genetically determined decrease in the function of the CYP2C19 isoenzyme in recommended doses (there is literature data indicating that patients with a genetically determined decrease in the function of the CYP2C19 isoenzyme are exposed to less systemic exposure to the active metabolite of clopidogrel and have a less pronounced effect of the drug, in addition, they may experience a higher incidence of cardiovascular complications after myocardial infarction compared to patients with normal function of the CYP2C19 isoenzyme).
Side Effects
Side Effects
Classification of the incidence of side effects (WHO): often (>1/100 and 1/1000 and 1/10,000 and <1/1000), very rare (<1/10,000).
From the central and peripheral nervous system: infrequently – headache, dizziness and paresthesia; rarely – vertigo; very rarely – disturbance of taste.
Mental disorders: very rarely – confusion, hallucinations.
From the cardiovascular system: very rarely – vasculitis, marked decrease in blood pressure, intracranial hemorrhage, ocular hemorrhage (conjunctival, in the tissue and retina), hematoma, nosebleeds, bleeding from the respiratory tract, gastrointestinal bleeding, fatal retroperitoneal hemorrhage, hemorrhages in the muscles and joints, hematuria.
From the respiratory system: very rarely – bronchospasm, interstitial pneumonitis.
From the digestive system: often – diarrhea, abdominal pain, dyspepsia; uncommon – gastric and duodenal ulcers, gastritis, vomiting, nausea, constipation, flatulence; very rarely – pancreatitis, colitis (including ulcerative or lymphocytic colitis), stomatitis, acute liver failure, hepatitis.
From the urinary system: very rarely – glomerulonephritis.
From the blood coagulation system: infrequently – prolongation of bleeding time.
From the hematopoietic system: infrequently – thrombocytopenia, leukopenia, neutropenia and eosinophilia; very rarely – thrombocytopenic thrombohemolytic purpura, severe thrombocytopenia (platelet count ≤30×109/l), agranulocytosis, granulocytopenia, aplastic anemia (pancytopenia), anemia.
From the skin and subcutaneous tissues: infrequently – skin rash and itching; very rarely – angioedema, urticaria, erythematous rash (associated with clopidogrel or acetylsalicylic acid); very rarely – bullous dermatitis (erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis), eczema and lichen planus.
From the musculoskeletal system: very rarely – arthralgia, arthritis, myalgia.
From the immune system: very rarely – anaphylactoid reactions, serum sickness.
From the laboratory parameters: very rarely – changes in liver tests, increase in serum creatinine concentration.
Other: very rarely – fever.
Interaction
Interaction
Warfarin: simultaneous use with clopidogrel may increase the intensity of bleeding, so the use of this combination is not recommended.
IIb/IIIa receptor blockers: the administration of IIb/IIIa receptor blockers together with clopidogrel requires caution in patients with an increased risk of bleeding (in case of trauma and surgery or other pathological conditions).
Acetylsalicylic acid: Acetylsalicylic acid does not alter the effect of clopidogrel, which inhibits ADP-induced platelet aggregation, but clopidogrel potentiates the effect of acetylsalicylic acid on collagen-induced platelet aggregation. However, simultaneous administration of acetylsalicylic acid 500 mg 2 times a day for 1 day with clopidogrel did not cause a significant increase in the bleeding time caused by taking clopidogrel. There may be a pharmacodynamic interaction between clopidogrel and acetylsalicylic acid, which leads to an increased risk of bleeding. Therefore, caution should be exercised when using them simultaneously. Although in clinical studies, patients received combination therapy with clopidogrel and acetylsalicylic acid for up to one year.
Heparin: According to a clinical trial conducted in healthy subjects, clopidogrel did not require a change in the dose of heparin and did not alter its anticoagulant effect. Concomitant use of heparin did not change the antiplatelet effect of clopidogrel. There may be a pharmacodynamic interaction between clopidogrel and heparin, which may increase the risk of bleeding, so the simultaneous use of these drugs requires caution.
Thrombolytics: The safety of combined use of clopidogrel, fibrin-specific or fibrin-non-specific drugs and heparin has been studied in patients with acute myocardial infarction. The incidence of clinically significant bleeding was similar to that observed in the case of combined use of thrombolytic agents and heparin with acetylsalicylic acid.
Nonsteroidal anti-inflammatory drugs (NSAIDs): In a clinical study in healthy volunteers, coadministration of clopidogrel and naproxen increased occult GI blood loss. However, due to the lack of interaction studies between clopidogrel and other NSAIDs, it is currently not known whether there is an increased risk of gastrointestinal bleeding when clopidogrel is taken with other NSAIDs. Therefore, the prescription of NSAIDs, including COX-2 inhibitors in combination with clopidogrel, should be used with caution. Other combination therapy: Since clopidogrel is metabolized to its active metabolite in part by the CYP2C19 system, the use of drugs that inhibit this system (for example, omeprazole) is not recommended.
A number of clinical studies have been conducted with clopidogrel and other concomitantly prescribed drugs to examine possible pharmacodynamic pharmacokinetic interactions, which have shown that:
When clopidogrel was used together with atenolol, nifedipine, or with both drugs at the same time, no clinically significant pharmacodynamic interaction was observed
simultaneous use of phenobarbital, cimetidine and estrogens did not have a significant effect on the pharmacodynamics of clopidogrel
the pharmacokinetic parameters of digoxin and theophylline did not change when they were used together with clopidogrel
Antacids did not reduce the absorption of clopidogrel
Phenytoin and tolbutamide can be used safely concomitantly with clopidogrel (CAPRIE study), although data from studies with human liver microsomes suggest that the carboxyl metabolite of clopidogrel may inhibit the activity of cytochrome P450 isoenzyme 2C9, which may lead to increased plasma concentrations of some drugs (phenytoin, tolbutamide and some NSAIDs), which are metabolized by the 2C9 isoenzyme of the cytochrome P450 family.
ACE inhibitors, diuretics, β-blockers, slow calcium channel blockers, lipid-lowering drugs, coronary vasodilators, lipid-lowering drugs (including insulin), antiepileptic drugs, hormone replacement therapy and GPIIb/IIIa receptor blockers: no clinically significant adverse interactions were identified in clinical studies.
Overdose
Overdose
An overdose of clopidogrel can lead to prolongation of bleeding time and hemorrhagic complications.
If bleeding is detected, appropriate treatment should be applied.
No antidotes for the pharmaceutical activity of clopidogrel have been found.
If rapid correction of prolonged bleeding time is necessary, platelet transfusion is recommended.
Storage conditions
Storage conditions
The drug should be stored out of the reach of children, in a dry, dark place at a temperature not exceeding 25°C.
Shelf life
Shelf life
3 years. Do not use after the expiration date stated on the package.
Manufacturer
Manufacturer
North Star NAO, Russia
Additional information
| Shelf life | 3 years. Do not use after the expiration date printed on the package. |
|---|---|
| Conditions of storage | The drug should be kept out of reach of children, dry and protected from light at a temperature not exceeding 25 ° C. |
| Manufacturer | North Star NAO, Russia |
| Medication form | pills |
| Brand | North Star NAO |
Other forms…
Related products
Buy Clopidogrel-SZ, 75 mg 28 pcs with delivery to USA, UK, Europe and over 120 other countries.