No products in the cart.
Clopidogrel, 75 mg 28 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
Prevention of thrombosis, Prevention of thromboembolism, Stenocardia, Prevention of heart attack, Prevention of stroke
Prevention of atherothrombotic complications:
- In patients with myocardial infarction (several days to 35 days old), ischemic stroke (7 days to 6 months old) or diagnosed peripheral artery occlusive disease.
- in patients with acute coronary syndrome:
- without ST-segment elevation (unstable angina or myocardial infarction without Q-wave), including patients who have had stenting for percutaneous coronary intervention (in combination with acetylsalicylic acid);
- with ST-segment elevation (acute myocardial infarction) with drug treatment and the possibility of thrombolysis (in combination with acetylsalicylic acid).
Prevent atherothrombotic and thromboembolic complications, including stroke, in atrial fibrillation (atrial fibrillation)
In patients with atrial fibrillation (atrial fibrillation) who have at least one risk factor for vascular complications, cannot take indirect anticoagulants, and have a low risk of bleeding (in combination with ASA).
Active ingredient
Active ingredient
Composition
Composition
the film-coated tablet contains:
Composition of the tablet core:
Active ingredient:
clopidogrel hydrosulfate – 97.87 mg (in terms of clopidogrel – 75.00 mg);
Auxiliary substances:
anhydrous lactose, 67.87 mg;
microcrystalline cellulose, 54.60 mg;
Crospovidone – 12.50 mg;
Glyceryl dibegenate – 7.50 mg;
Tarnish – 7.50 mg;
Sodium starch glycolate – 2.50 mg.
Composition of the tablet shell:
Opadray II pink (85G240008) – 8.00 mg (polyvinyl alcohol – 44.00%, titanium dioxide – 19.50%, macrogol 3350 – 12.35%, talc – 20.00%, lecithin (soy) – 3.50%, iron oxide red dye – 0.50%, iron oxide yellow dye – 0.15%).
How to take, the dosage
How to take, the dosage
Ingestion, regardless of meals.
Patients with normal isoenzyme activity CYP2C19
Myocardial infarction, ischemic stroke, or diagnosed peripheral artery occlusive disease.
1 film-coated tablet (75 mg) once daily.
In patients with myocardial infarction (MI), treatment can be started from the first days to day 35 of MI, and in patients with ischemic stroke (IS), from 7 days to 6 months after IS.
Acute coronary syndrome without ST-segment elevation (unstable angina pectoris, myocardial infarction without Q-wave)
. Treatment with clopidogrel should be started with a single loading dose of 300 mg and then continued with a dose
75 mg once daily (in combination with ASA at doses of 75-325 mg daily). Since the use of higher doses of ASA is associated with an increased risk of bleeding, the recommended dose of ASA for this indication should not exceed 100 mg. The optimal duration of treatment for this indication has not been officially determined. The results of clinical studies confirm the appropriateness of the drug up to 12 months after the development of acute coronary syndrome without ST-segment elevation. Maximum favorable effect is observed by the third month of treatment.
Acute coronary syndrome with ST-segment elevation (acute ST-segment elevation myocardial infarction)
Clopidogrel Clopidogrel is administered as a single dose of 75 mg once daily with an initial single loading dose of clopidogrel 300 mg in combination with ASA and thrombolytics (or without thrombolytics). In patients over 75 years old, treatment with clopidogrel should be started without its loading dose. Combination therapy should be started as soon as possible after the onset of symptoms and continued for at least four weeks. The effectiveness of combined use of clopidogrel and acetylsalicylic acid beyond 4 weeks in this indication has not been studied.
Atrial fibrillation (atrial fibrillation)
Clopidogrel should be taken once daily in a dose of 75 mg. In combination with clopidogrel, ASK should be started and then continued
(75-100 mg/day).
Missing another dose
Patients with genetically determined reduced CYP2C19 isoenzyme activity
Slow CYP2C19-metabolizer status is associated with decreased antiaggregant effect of clopidogrel. A higher dose regimen (600 mg – loading dose, then 150 mg once daily) in slow metabolizers increases the antiaggregant effect of clopidogrel (see section “Pharmacokinetics”). However, the optimal dosing regimen for patients with decreased CYP2C19 isoenzyme activity has not yet been established in clinical trials.
Special patient groups
.Em>Patients in the elderly
No dose adjustment is required.
Patients of pediatric age
There is no experience with the drug in children.
Patients with impaired renal function
Patients with impaired renal function. After repeated administration of clopidogrel at dose 75 mg/day in patients with severe renal impairment (creatinine clearance from 5 to 15 ml/min) inhibition of ADP-induced platelet aggregation (25%) was lower compared to that in healthy volunteers, but prolongation of bleeding time was similar to that in healthy volunteers receiving clopidogrel at dose 75 mg daily. In addition, all patients had good tolerability of the drug.
Patients with hepatic impairment
After 10 days daily use of clopidogrel at a daily dose
75 mg, inhibition of ADP-induced platelet aggregation in patients with severe hepatic impairment was similar to that in healthy volunteers. The mean bleeding time was also comparable in both groups.
Patients of different racial backgrounds
The prevalence of CYP2C19 isoenzyme gene alleles responsible for the intermediate and reduced metabolism of clopidogrel to its active metabolite varies among different ethnic groups (see section “Pharmacogenetics”). Only limited literature data are available for members of the Mongoloid race to assess the clinical relevance of CYP2C19 isoenzyme genotyping in predicting the development of ischemic complications.
Interaction
Interaction
Orderal anticoagulants: concomitant administration of clopidogrel and oral anticoagulants may increase the intensity of bleeding, in connection with this combination is not recommended. Clopidogrel has no effect on warfarin pharmacokinetics and does not change the international normalized ratio (INR) in patients taking warfarin for a long time. However, concomitant administration of warfarin with clopidogrel may increase the risk of bleeding due to the independent effects of these drugs on hemostasis.
The administration of glycoprotein IIb/IIIa inhibitors together with clopidogrel requires caution in patients with increased risk of bleeding (in trauma and surgery or other pathological conditions) (see section “Special Precautions”).
Acetylsalicylic acid: ASA does not change the effect of clopidogrel inhibiting ADP-induced platelet aggregation, but clopidogrel increases the effect of ASA on collagen-induced platelet aggregation. However, concomitant administration of 500 mg of ASA twice daily for 1 day with clopidogrel did not significantly increase clopidogrel-induced bleeding time. Pharmacodynamic interaction is possible between clopidogrel and ASA, which leads to an increased risk of bleeding. Therefore, caution should be exercised when using them concomitantly, although in clinical trials patients received combined therapy with clopidogrel and ASA for up to one year.
Heparin: According to a clinical study conducted with healthy volunteers, the administration of clopidogrel did not require a change in heparin dose and did not alter its anticoagulant effect. Co-administration of heparin did not alter the inhibitory effect of clopidogrel on platelet aggregation. There is a possible pharmacodynamic interaction between clopidogrel and heparin, which may increase the risk of bleeding, so the simultaneous use of this combination requires caution.
Thrombolytics:The safety of concomitant use of clopidogrel, fibrin-specific or fibrin-unspecific thrombolytics and heparin has been studied in patients with acute myocardial infarction. The incidence of clinically significant bleeding was similar to that observed when thrombolytics and heparin were used together with ASA.
Non-steroidal anti-inflammatory drugs (NSAIDs):In a clinical study involving healthy volunteers, coadministration of clopidogrel and naproxen increased latent blood loss through the gastrointestinal tract. However, due to the lack of studies on the interaction of clopidogrel with other NSAIDs, it is currently unknown whether there is an increased risk of gastrointestinal bleeding when clopidogrel is taken together with other NSAIDs. Therefore, the use of NSAIDs, including COX-2 inhibitors, in combination with clopidogrel should be used with caution.
Selective serotonin reuptake inhibitors (SSRIs): because SSRIs impair platelet activation and increase the risk of bleeding, coadministration of SSRIs with clopidogrel should be performed with caution.
CYP2C19 inhibitors
Because clopidogrel is metabolized to its active metabolite partially by the CYP2C19 isoenzyme, use of drugs that inhibit this isoenzyme may result in decreased levels of the active metabolite clopidogrel and decrease its clinical effectiveness. The clinical significance of this interaction has not been established. As a precautionary measure, it is recommended to avoid co-administration of clopidogrel with drugs that inhibit CYP2C19 isoenzyme (omeprazole, esomeprazole, fluvoxamine, fluoxetine, moclobemide, voriconazole, fluconazole, ticlopidine, ciprofloxacin, cimetidine, carbamazepine, oxcarbazepine, chloramphenicol).
Proton pump inhibitors
If it becomes necessary to prescribe proton pump inhibitors concomitantly with clopidogrel, the drug with the least CYP2C19 inhibition (pantoprazole or lanzoprazole) should be used.
Other combination therapy
A number of clinical studies have been conducted with clopidogrel and other concomitant medications to examine possible pharmacodynamic and pharmacokinetic interactions, which showed that:
– no clinically significant pharmacodynamic interactions were observed when clopidogrel was coadministered with atenolol, nifedipine, or both drugs simultaneously;
– the concurrent use of phenobarbital, cimetidine, and estrogen had no significant effect on the pharmacodynamics of clopidogrel;
– the pharmacokinetic parameters of digoxin and theophylline were not altered by their concomitant use with clopidogrel;
– antacids did not decrease absorption of clopidogrel;
– According to the CAPRIE study, phenytoin and tobutamide can be safely used with clopidogrel. It is unlikely that clopidogrel can affect the metabolism of other drugs, such as phenytoin and tobutamide, as well as NSAIDs that are metabolized by CYP2C19 isoenzyme;
– In clinical studies no clinically significant adverse interactions of clopidogrel with angiotensin-converting enzyme inhibitors, diuretics, beta-adrenoblockers, “slow” calcium channel blockers, hypolipidemic agents, coronary vasodilators, hypoglycemic agents (including insulin) have been revealed.including with insulin), antiepileptic agents, preparations for hormone replacement therapy.
Special Instructions
Special Instructions
When treating with clopidogrel, especially during the first weeks of treatment and/or after invasive cardiac procedures/surgery, patients should be closely monitored for signs of bleeding, including hidden bleeding.
Because of the risk of bleeding and hematologic adverse effects (see section “Side effects”), if clinical symptoms indicating possible bleeding occur during treatment, clinical blood counts, activated partial thromboplastin time (APTB), platelet counts, platelet functional activity parameters and other necessary tests should be performed urgently.
. Clopidogrel, as well as other antiplatelet agents, should be used with caution in patients who are at increased risk of bleeding due to trauma, surgery or other pathological conditions, as well as in patients receiving ASA, NSAIDs including COX-2 inhibitors, heparin or glycoprotein IIb/IIIa inhibitors, SSRIs and thrombolytics.
The co-administration of clopidogrel with warfarin may increase the intensity of bleeding (see section “Interaction with other medicinal products”), so caution should be exercised when co-administering clopidogrel and warfarin.
If the patient is to have elective surgery and there is no need for antiplatelet effects, clopidogrel should be discontinued 7 days prior to surgery.
Clopidogrel prolongs bleeding time and should be used with caution in patients with conditions that predispose to bleeding (especially gastrointestinal and intraocular). Drugs that may cause gastrointestinal mucosal damage (such as ASA, NSAIDs) in patients taking clopidogrel should be used with caution.
Patients should be warned that it may take longer to stop bleeding when clopidogrel is taken (alone or in combination with ASA) and that they should inform their physician if they have unusual bleeding (in location or duration). Patients should tell their doctor (including their dentist) about clopidogrel before any upcoming surgery and before starting any new medication.
Very rare cases of thrombotic thrombocytopenic purpura, characterized by thrombocytopenia and microangiopathic hemolytic anemia, accompanied by neurological disorders, impaired renal function and fever, have been reported after clopidogrel use (sometimes even short-term) and are a potentially life-threatening condition requiring immediate treatment, including plasmapheresis.
Hypatic function should be monitored during treatment. In severe liver lesions, be aware of the risk of hemorrhagic diathesis.
The drug should be used with caution in patients with impaired renal function.
The use of clopidogrel is not recommended for acute stroke less than 7 days old (because there are no data on its use in this condition).
In patients with recent transient cerebral haemorrhage or stroke who have a high risk of recurrent ischemic complications, the combination of ASA and clopidogrel increases the incidence of major bleeding. Therefore, such combination therapy should be used with caution and only if there is proven clinical benefit from its use.
Cases of acquired hemophilia have been reported when taking clopidogrel. If an isolated increase in ACTV is confirmed, with or without bleeding, the possibility of acquired hemophilia should be considered. Patients with a confirmed diagnosis of acquired hemophilia should be monitored and treated by specialists in this disease and discontinue clopidogrel.
. In patients with low CYP2C19 isoenzyme activity, the use of clopidogrel at recommended doses produces less of the active metabolite clopidogrel and its antiaggregant effect is less pronounced; therefore, a higher incidence of cardiovascular complications may occur with the commonly recommended doses of clopidogrel in acute coronary syndrome or percutaneous coronary intervention than in patients with normal CYP2C19 isoenzyme activity. Tests are available to determine the CYP2C19 genotype. These tests can be used to assist in the choice of therapeutic strategy. The use of higher doses of clopidogrel in patients with low CYP2C19 isoenzyme activity is being considered (see subsection “Pharmacogenetics” of section “Pharmacological properties”, sections “Caution”, “Dosage and administration”).
The patients’ history of allergic and/or hematological reactions to other thienopyridine derivatives (ticlopidine, prasugrel) should be specified since there have been described cases of cross-allergic and/or hematological reactions between thienopyridines (see section “Adverse effects”). Patients who have previously had allergic and/or hematological reactions to other thienopyridine derivatives require close monitoring during the entire period of therapy to detect signs of hypersensitivity to clopidogrel.
Clopidogrel should not be taken in patients with rare hereditary galactose intolerance, lactose deficiency and glucose-galactose malabsorption syndrome.
Influence of the drug on performance of potentially hazardous activities requiring increased concentration and rapid psychomotor reactions
Clopidogrel has no significant effect on the ability to drive or engage in potentially hazardous activities.
Contraindications
Contraindications
Cautious
Side effects
Side effects
The following WHO classification is used to estimate the incidence of adverse events:
Very common (⥠1/10)
Frequent (⥠1/100 and < 1/10)
Infrequent (⥠1/1000 and < 1/100)
Rarely (⥠1/10,000 and < 1/1000)
Very rare (⥠1/10,000)
The frequency is unknown (it is not possible to determine the incidence of the adverse event from the available data).
Blood and lymphatic system disorders:
infrequent – thrombocytopenia, leukopenia, eosinophilia;
rare – neutropenia, including severe neutropenia;
very rare – thrombotic thrombocytopenic purpura, aplastic anemia, pancytopenia, agranulocytosis, severe thrombocytopenia, granulocytopenia, anemia;
frequency unknown – acquired hemophilia A.
Income immune system disorders:
very rare – anaphylactic reactions, serum sickness;
frequency unknown – cross-sensitivity to other thienopyridines (e.g., ticlopidine, prasugrel);
Nervous system disorders:
infrequent – intracranial hemorrhage (several cases with fatal outcome), headache, paresthesias, dizziness;
very rare – disorders of taste perception.
Mental disorders:
very rarely – confusion, hallucinations.
Visual disorders:
infrequent – retinal hemorrhage, conjunctival hemorrhage, hemophthalmus.
Hearing organ and labyrinth disorders:
rarely – vertigo.
Skin and subcutaneous tissue side:
often – “bruising” (localized subcutaneous hemorrhages);
infrequently – skin rash and itching, thrombocytopenic purpura;
very rare – angioedema, erythematous rash, urticaria, bullous dermatitis (toxic epidermal necrolysis, Stevens-Johnson syndrome, erythema multiforme), eczema, lichen planus, drug hypersensitivity syndrome, drug rash with eosinophilia and systemic manifestations (DRESS syndrome), exfoliative rash.
Renal and urinary tract disorders:
infrequent – hematuria;
very rare – glomerulopathy (includingincluding glomerulonephritis), hypercreatininemia.
Musculoskeletal and connective tissue disorders:
very rarely – muscle and joint hemorrhage, arthritis, arthralgia, myalgia.
From the gastrointestinal tract:
very common – gastrointestinal bleeding, diarrhea, abdominal pain, dyspepsia;
infrequent – gastric and duodenal ulcer, gastritis, vomiting, nausea, constipation, flatulence;
rare – retroperitoneal hemorrhage;
very rare – gastrointestinal bleeding and retroperitoneal hemorrhage with fatal outcome, pancreatitis, colitis (including ulcerative colitis or lymphocytic colitis), stomatitis.
Hepatic and biliary tract disorders:
very rarely – acute liver failure, hepatitis (non-infectious).
As to the respiratory system, thoracic and mediastinal organs:
often – nasal bleeding;
very rarely – respiratory bleeding (hemoptysis, pulmonary bleeding), interstitial pneumonitis, bronchospasm, eosinophilic pneumonia.
Cardiovascular system disorders:
often – hematoma;
very rarely – severe bleeding, bleeding from the operating wound, vasculitis, decreased blood pressure.
Laboratory and instrumental data:
infrequent – prolongation of bleeding time, decreased number of neutrophils and/or platelets in peripheral blood, impaired liver function tests, increased plasma creatinine concentration.
General disorders and disorders at the site of administration:
often – bleeding from the site of vascular puncture;
very rarely – increase in body temperature.
Overdose
Overdose
Symptoms:increased bleeding time with subsequent complications in the form of bleeding development.
Treatment:In case of bleeding, appropriate treatment measures are required, platelet transfusion. There is no specific antidote.
Similarities
Similarities
Additional information
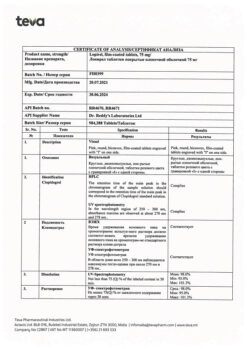
| Manufacturer | Izvarino Pharma, Russia |
|---|---|
| Medication form | pills |
| Brand | Izvarino Pharma |
Other forms…
Related products
Buy Clopidogrel, 75 mg 28 pcs with delivery to USA, UK, Europe and over 120 other countries.